Use cases for the mapping
Authoring a SNOMED CT Drug Extension
- The national extension wants to represent authorised (real/branded) medicinal products in a SNOMED CT drug extension as RCDs, based on the authorised product information (section 3 of SmPC)
- The authorised product information uses EDQM dose forms
Therefore: a map from EDQM dose forms to SNOMED CT dose forms significantly helps the authoring of the RCDs, because “pharmaceutical dose form” is a definitional attribute of an RCD
National use of international concepts in patient care
- An organisation wants to map concepts from a national or local medication product dictionary (MPD) to international SNOMED CT clinical drugs for the patient medication process - prescribing/dispensing/administration/recording
“Prescription” uses abstract concepts (from the international edition) but these must be matched to a local authorised product for dispensing/administration - Many or all of the concepts in the MPD use EDQM dose forms as their dose form
Therefore: a map from EDQM dose forms to SNOMED CT dose forms significantly helps the mapping because “pharmaceutical dose form” is a definitional attribute of a SNOMED CT clinical drug and is usually a stated attribute of the concept in the MPD. By mapping at attribute level, much of the concept level mapping can be “automated” (at least initially)
International interoperability of medication information
- An organisation wants to map concepts from a national or local medication product dictionary (MPD) to international SNOMED CT clinical drugs for interoperability purposes, such as the sharing of medication lists for cross-border patient-care, pharmacovigilance and clinical research
- Many or all of the concepts in the MPD use EDQM dose forms as their dose form
Therefore: a map from EDQM dose forms to SNOMED CT dose forms significantly helps the mapping because “pharmaceutical dose form” is a definitional attribute of a SNOMED CT clinical drug and is usually a stated attribute of the concept in the MPD. By mapping at attribute level, much of the concept level mapping can be “automated” (at least initially)
Decision support
- An organisation wants to manage medication decision support data using “international” medication concepts, so uses SNOMED CT and its structures, either directly or indirectly
- It then needs to map from local MPD to SNOMED CT for implementation (if the local MPD is not already mapped to SNOMED CT)
- It may also want to “stream in” IDMP data for processing in its decision support system (as in UC4)
Therefore: a map from EDQM dose forms to SNOMED CT dose forms significantly helps the mapping because “pharmaceutical dose form” is a definitional attribute of a SNOMED CT clinical drug and is usually a stated attribute of the concept in the MPD. By mapping at attribute level, much of the concept level mapping can be “automated” (at least initially)
Note: in many ways, this UC is another view on all of the previous use cases, since it involves mapping from local/national MPD to SNOMED CT, using authorised product data etc.
IDMP / UNICOM
- An organisation wants to map data from an IDMP-compliant regulatory database of medicinal products to SNOMED CT concepts
- An IDMP-compliant regulatory database should EDQM dose forms as their dose form
Therefore: a map from EDQM dose forms to SNOMED CT dose forms significantly helps the mapping because “pharmaceutical dose form” is a core part of SNOMED CT medicinal product information
Note: in many ways, this UC is another view on all three of the previous use cases, but we document it separately because of the importance of UNICOM, especially to our European members
Aims and Objectives
To produce as close as possible to a 1..1 exact semantic match mapping between the EDQM Pharmaceutical Dose Forms as source and the SNOMED CT descendants of 736542009 | Pharmaceutical dose form (dose form) | to support the use cases described above.
When an exact match is not possible, further discussions will be undertaken with the relevant parties and guidance will be offered to users of the map.
Mapping Policy and Principles
Scope
Map source: EDQM Pharmaceutical Dose Forms
Active PDFs at 2021-09-07 that are not ‘Veterinary only’
A "snapshot" full download of EDQM PDF data was taken via the EDQM API
Map target: SNOMED CT 20210731 international edition
Active concepts that are < 736542009 | Pharmaceutical dose form (dose form) |
Notes:
Definition of a pharmaceutical dose form: “the physical manifestation of a Medicinal Product that contains the active ingredient(s) and/or inactive ingredient(s) that are intended to be delivered to the patient”
Based on the use cases, PDFs that have been inactivated in EDQM may still be used in product SmPCs; these will require a historical association to an active PDF. This should be considered once the initial scope has been completed
Patient Friendly Terms (PFT) are currently excluded; these may be used in product labelling but are not used as a definitional attribute for a medicinal product.
Authorised dose forms (those that may be used in section 3 of an SmPC in Europe) include Combined Pharmaceutical Dose Forms, Combined Terms and Combination Pack concepts as well as PDFs. In order to fulfil use case 1, these concepts may require addition to SNOMED CT but currently they are out of scope.
We are not mapping the EDQM and SNOMED CT dose form attribute values, because for EDQM, these are not definitional so might give us misleading results if we then tried to do an "automatic" map based on these. However, we are noting when the attribute values between two concepts that give an exact map based on their definition do not have an exact attribute value map.
Cardinality
The aim is to get 1..1 semantic maps wherever possible and to provide guidance in all situations where that is not the case.
Association/Correlation
The aim is for the source and target to be "equivalent" (i.e. an exact semantic match)
But there will be some maps that are not exact, but which we wish to make and to note that they have a different association.
Narrower (than): Use this association when the meaning of the SNOMED CT concept is narrower than the meaning of the EDQM concept
- Another way of saying this is that this is a "broad-to-narrow" map (from EDQM source to S-CT target); this is a 'narrower' mapping, because you're mapping to a narrower meaning
For example, a mapping between "Intravesical solution/solution for injection" in EDQM to "Conventional release intravesical solution" in S-CT is "narrower" as the EDQM concept appears to suggest two quite different methods of administration are possible, whereas the S-CT concept is specifically a solution - Some 'Narrower target' maps may be made, in situations where the EDQM concept is inappropriate for inclusion in SNOMED CT but alternative more granular concepts are available. These may give a 1..* cardinality.
Broader (than): Use this association when the meaning of the SNOMED CT concept is broader than the meaning of the EDQM concept
- Another way of saying this is that this is a "narrow-to-broad" map (from EDQM source to S-CT target); this is a 'broader' mapping, because you're mapping to a broader meaning
For example, a mapping between "Capsule, soft" in EDQM to "Conventional release oral capsule" in S-CT is "broader" as the S-CT concept encompasses both hard and soft capsule types - Some 'Broader target’ maps may be made; these are likely to give a *..1 cardinality when the mapping is considered in its entirety
In all cases where the final mapping is 'Broader target’ or 'Narrower target', implementation guidance will be provided.
Notes on "Semantic Match"
Source terminology: EDQM Pharmaceutical Dose Forms
Definition and Comment
In addition to the name of the PDF concept, the text definition of the term important to understand its meaning. BUT not every part of the definition can be considered for the semantic and for mapping.
Local or systemic effect
In the concept below "to obtain a local effect" is stated. The definition of a PDF (see above) is concerned with it being the thing that formulates or encapsulates or contains the active and inactive ingredient substances either at the point of supply (the manufactured dose form) or at the point of administration (the administrable dose form); there should be no consideration of what happens to the active substance(s) once the administration has been made and therefore no consideration of what or where their effect is. It is not possible to state accurately for any one medicinal product whether its effect (either therapeutically desired or undesired) will be "local" or "systemic" based on its dose form; for example, there are oral tablets that have no systemic effect (e.g. nystatin). Therefore, if "for local effect" (or "for systemic effect") is part of either the definition or the comment for an EDQM PDF concept, it will not be deemed part of the semantic of the concept for mapping.
But there may be useful information in the "Comment" section that may need to be considered for mapping:
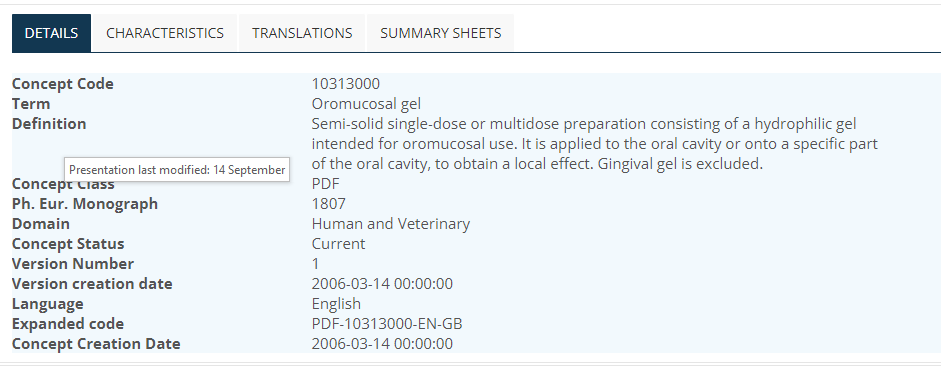
Although the "Comment" section is primarily aimed as “implementation guidance” for marketing authorisation applicants to guide them when they are selecting a PDF to use for their product, it may give information that “clarifies” the definition, so is something to consider when making a mapping. For example, sometimes, the "Comment" section will describe what the concept is NOT as well as what it is, which may be important when considering a mapping. However, statements like “Sublingual sprays are excluded“ are guidance for manufacturers to avoid ambiguity when they are selecting dose forms for products, not statements about the intended site concept itself. The intent is that “if the product is a sublingual spray, give it a sublingual spray dose form, DON’T give it a “oromucosal spray” dose form (just because the sublingual site “is part of” the oromucosal“) (C Jarvis 2021-10-21) THEREFORE this makes the EDQM “oromucosal” similar to the SNOMED CT “oromucosal” – both act as grouper concepts. A 1..1 map can therefore be made between the oromucosal, sublingual and buccal concepts in EDQM and SNOMED CT without being concerned by these exclusion statements “altering” concept meaning.
EDQM
Characteristic values
The characteristic values for a PDF should also be examined whilst remembering that currently, the attributes of a PDF concept in EDQM are NOT definitional for the concept.
Definitions for the various values that can be used in the characteristics can be found at:
Prolonged release
The definition of "prolonged release" is clear that this is "achieved by a special formulation design and/or manufacturing method" - and therefore, by implication, not by the substance. Therefore products containing substances like "haloperidol decanoate" or "insulin isophane" or "insulin zinc suspension" should not have prolonged release dose forms as it is the modification of the substance that makes its release longer than the unmodified or simply modified substance, not the dose form.
Target terminology: SNOMED CT Pharmaceutical Dose Forms
In SNOMED CT, the fully specified name (FSN) ‘unambiguously’ represents the meaning of the concept. In addition:
- For "defined" concepts: the set of defining relationships sufficiently represents the logical meaning of the FSN
- For "primitive" concepts: the set of relationships represents part of the logical meaning of the FSN (the totality of the meaning is captured in the FSN)
There are some text definitions, especially for primitive concepts used as attributes in the concept model. For example, the "method of administration" attribute value concepts have a textual definition.
The (poly)hierarchy of concepts should reflect the relationships, which themselves represent the concept and therefore should be correct for the concept.
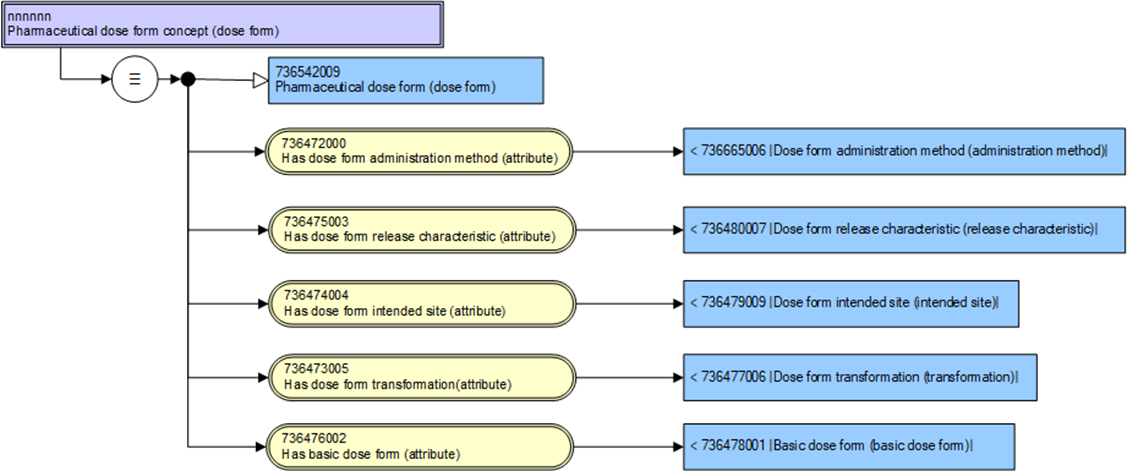
SNOMED CT PDFs are “qualifier values” because they are used as attributes in the medicinal product concept model, but they are still full concepts in their own right.
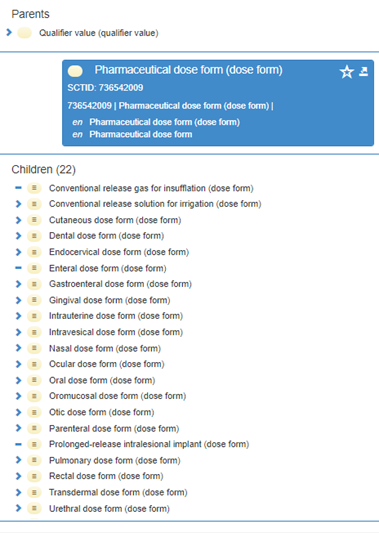
The parent (grouping) concept 736542009 | Pharmaceutical dose form (dose form) | has no attributes (and is therefore marked as “primitive”) but the child concepts - the PDF concepts themselves, have a concept model.
Example (stated view):
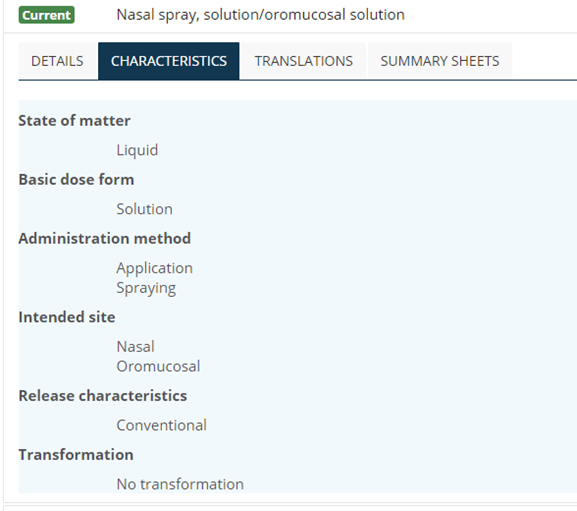
Although polyhierarchical, the SNOMED CT browser will display concepts in the inferred view in a hierarchy based on the intended site of the dose form, with a grouper concept acting as the parent:
Not all PDFs in SNOMED CT are “fully defined”. Two concepts cannot share the same logical definition and both be fully (logically) defined, so there are currently some primitive PDFs that have to be child concepts of fully defined concepts because they have extra meaning in their FSN. There ay also be some primitive siblings:
Transform Dose Forms
As a general principle. if there is a PDF that undergoes a transform to another PDF (e.g. "Powder for intravesical solution") whose transformed dose form (the administrable dose form) would be "Intravesical solution", the transformed PDF concept should be available in SNOMED CT International content even if there are no CDs that directly require it. Some extensions may model "administrable CDs" or equivalent concepts and require the administrable dose form.
Mapping Process
Cardinality: In the initial work, we will note when the map being made is 1..1 or 1..*. Only when we have completed the mapping will we be able to see the overall set of maps and then be able to confirm cardinalities.
Association/Correlation: Note the association for each map made.
Specific points from 23 September meeting:
13047000: Solution for suspension for injection – sounds like part of a “kit”. No products for now; agree to not consider until we absolutely need to
Lyophilisate– not included for injectable products as has no clinical effect, whereas for oral products it does
Oral administration – dose form delivers primarily to the stomach/duodenum for the intended site; Sublingual administration - dose form delivers primarily under the tongue for the intended site (and therefore misses first pass metabolism)
Mapping of "drops" dose forms
Specific points from 7 October meeting:
Yes, sometimes it feels like a jigsaw puzzle, but we are working at it together
Is it possible to have a longer meeting at any point (say 90 minutes) to help with the complexity of the subject matter? We'll consider if that's possible
Thoughts to come back to - the open world assumption in SNOMED CT for dose forms
Specific points from 21 October meeting:
Multiple intended site dose forms - should be added to SNOMED CT when we have products that require them, and the appropriate administrable dose form should be created in SNOMED CT even if there are no products using this directly….since some countries/implementations will be representing the administrable concept as well as the manufactured concept. Example for this is “Powder for intravesical solution/solution for injection” with its administrable dose form of “Intravesical solution/solution for injection”
Having the multiple intended site dose forms in SNOMED CT will mean we can make 1..1 associations for these EDQM concepts to SNOMED CT concepts, making the mapping implementation easier.
From Chris J: The EDQM “oromucosal” concept originated as a header term when the dose forms were published as a list. The statements like “Sublingual sprays are excluded“ are guidance for manufacturers to avoid ambiguity when they are selecting dose forms for products, not statements about the intended site concept itself. The intent is that “if the product is a sublingual spray, give it a sublingual spray dose form, DON’T give it a “oromucosal spray” dose form (just because the sublingual site “is part of” the oromucosal“)
This makes the EDQM “oromucosal” similar to the SNOMED CT “oromucosal” – both act as grouper concepts. We can therefore make 1..1 matches between the oromucosal, sublingual and buccal concepts in EDQM and SNOMED CT without being concerned by these exclusion statements “altering” concept meaning








4 Comments
François Macary
Julie M. James, in the association/correlation section's current wording, one can infer what "narrower than" and "broader than" mean by looking at your comment on the potential resulting cardinality (1..* for the former) and (*..1) for the latter. However I think that stressing the explicit definition would spare the group misinterpretations, especially for non-native English speakers lacking mapping experience. I have not seen the definition elsewhere on our Confluence space.
Something like that:
Linda Bird
That's a great point François Macary - definitely something worth clarifying. I think the definition is actually the opposite of what you're suggesting. Based on the W3C's SKOS model, the association between a map source and map target actually refers to the target in relation to the source. So in this case, "Narrower" means that SNOMED CT (the target) is narrower than the EDQM term. And "Broader means that SNOMED CT (the target) is broader than the EDQM term. So I would define them as follows:
Based on the W3C's SKOS model, the association between a map source and map target actually refers to the target in relation to the source. So in this case, "Narrower" means that SNOMED CT (the target) is narrower than the EDQM term. And "Broader means that SNOMED CT (the target) is broader than the EDQM term. So I would define them as follows:
I hope this makes sense. Julie - If you agree with this, then (as per François's suggestion) it may be worth adding this clarification above.
Thanks! Linda.
François Macary
Yes, the definition you propose, Linda Bird is the one we were used to, in particular with our experience of FHIR terminology services.
Somehow, I had misinterpreted the cardinality comment bit, and Olivier Bouxand I, had hesitations each time we came back to the mapping. Now I see that Olivier had the right one.
Adding your explicit definition will definitely avoid any risk of misinterpretations.
Thank you.
Julie M. James
I've updated the mapping guidance with some additional text and an example for each