Overview
The substance redesign project has made a number of decisions regarding the representation of a substance and its modifications.
- Modification indicates that the concept is a structural modification of another concept, where the intrinsic physicochemical properties of the substance do not change.
- The intrinsic physicochemical properties do not encompass non-intrinsic characteristics of a substance, such as toxicity, bioavailability, or extended release (in the case of medicinal products).
- Modification concepts that exist to group modifications or derivatives of a specific substance are not to be created.
- Metabolite groupers in the substances hierarchy are considered roles. No further concepts of this format will be created in the |Substance| hierarchy. Existing concepts will only be retained where there is a specific requirement and will be modeled as a child of |Metabolite (substance)|. See Metabolites.
- Structural groupers that reference modifications as a chemical group will be retained. Where the structural group name also refers to a specific chemical the grouper will be re-termed “Substance with X structure”. See relative section Substance Groupers Based on Structure.
- There is no requirement to introduce a new semantic tag in order to distinguish concepts representing a substance or its modifications from any other type of concept in the |Substance| hierarchy.
Modeling
| Semantic tag | (substance) |
|---|---|
| Definition status | Primitive |
Attribute: Is modification of | Range: <105590001 |Substance (substance)| |
Exemplar
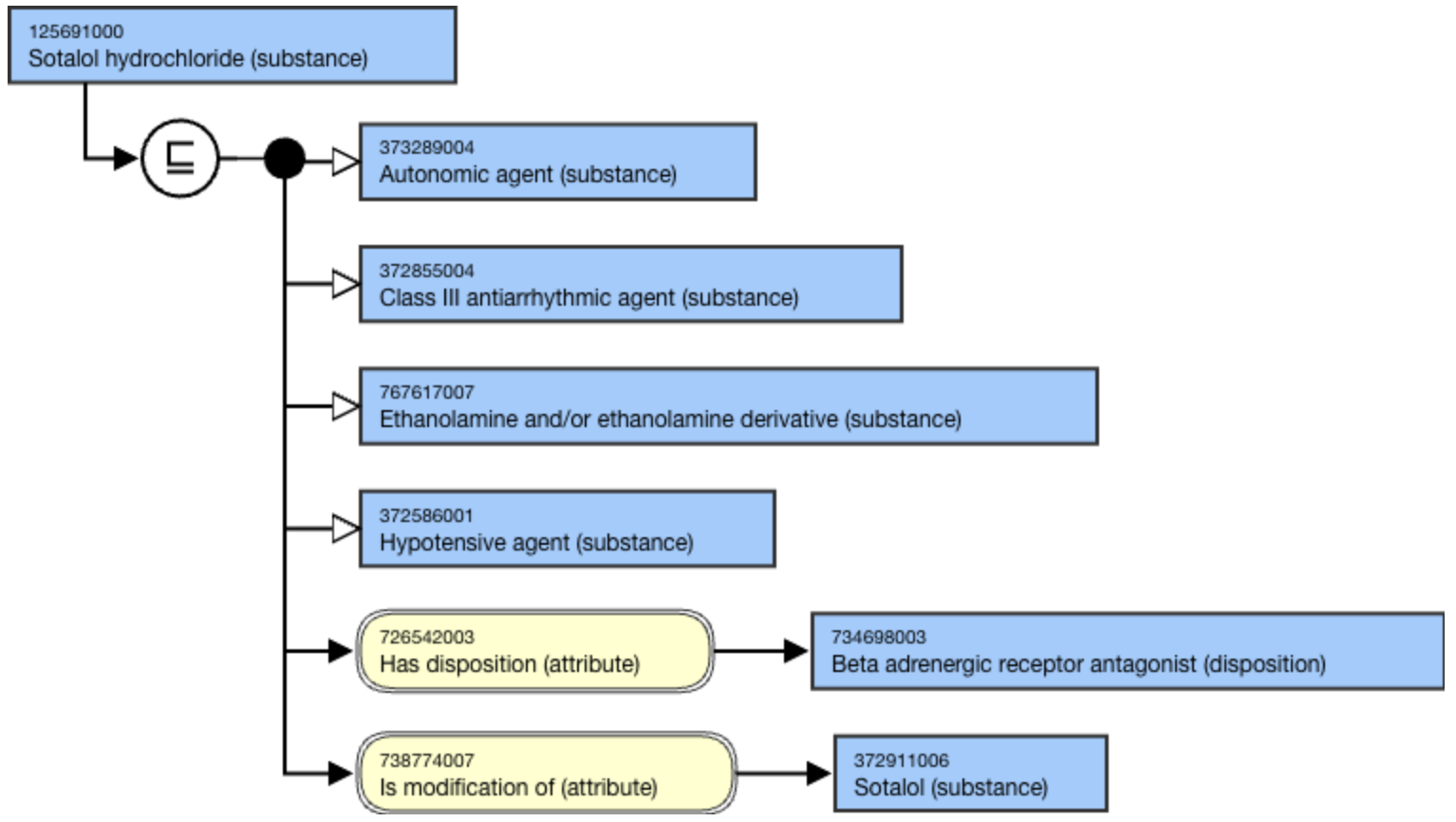
The following illustrates the stated view of 125691000 |Sotalol hydrochloride (substance)|:
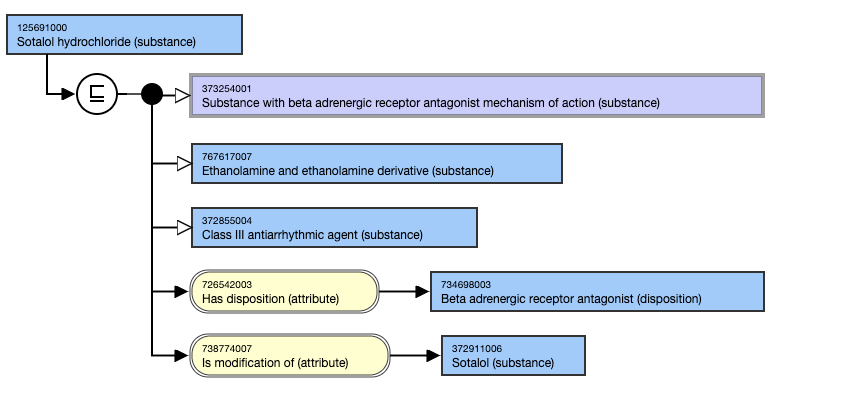
The following illustrates the inferred view for 125691000 |Sotalol hydrochloride (substance)|:
Guidelines for the use of 738774007 | Is modification of (attribute)|
Substances may have zero to many Is modification of attribute(s).
For example,
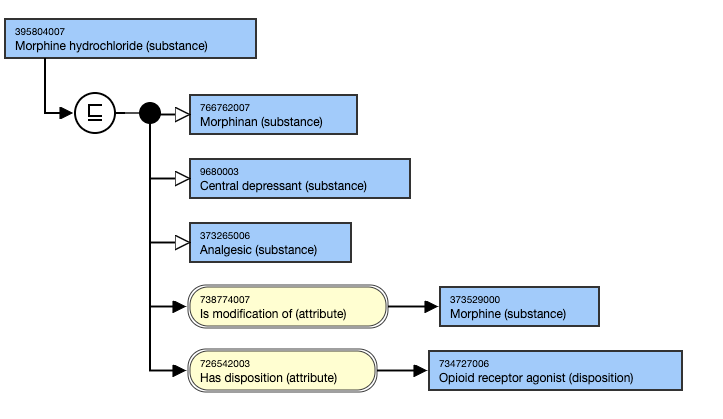
- Morphine hydrochloride (substance)
For example,
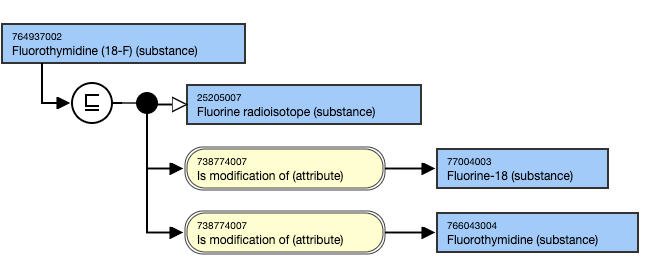
- Fluorothymidine (18-F) (substance)
For example,
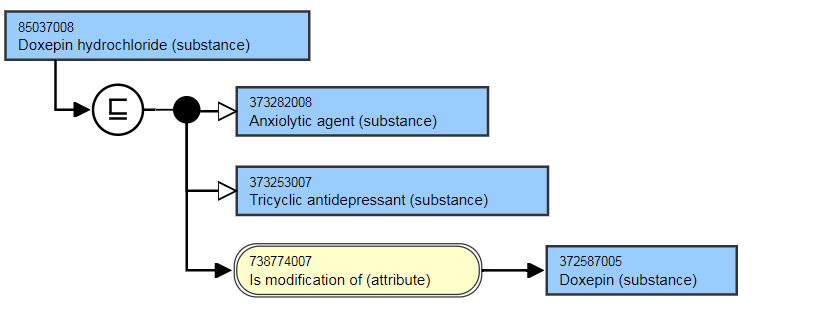
- Doxepin hydrochloride (substance)
Hydrates have an 738774007 | Is modification of (attribute)| relationship to the unspecified salt.
For example,
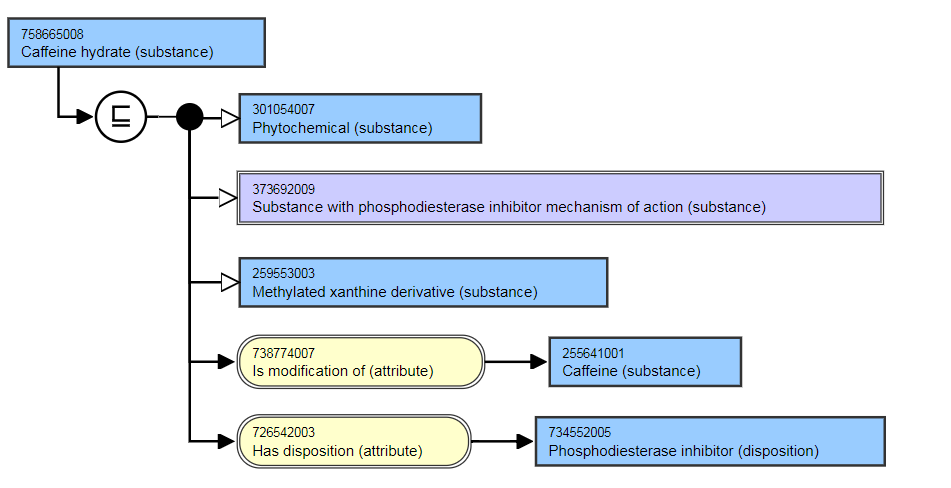
- Caffeine hydrate (substance) IS MODIFICATION OF Caffeine (substance).
This is the stated view:
This is the inferred view:
For example,
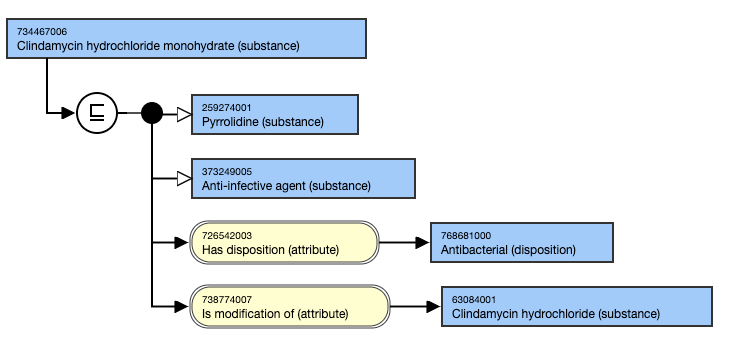
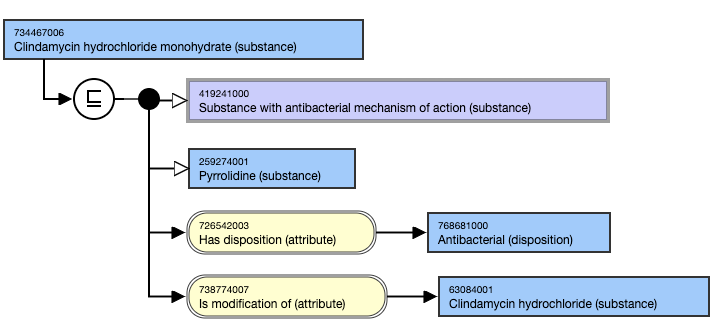
- Clindamycin hydrochloride monohydrate (substance) IS MODIFICATION OF Clindamycin hydrochloride (substance).
- Clindamycin hydrochloride monohydrate (substance) IS MODIFICATION OF Clindamycin hydrochloride (substance).
This is the stated view:
This is the inferred view:
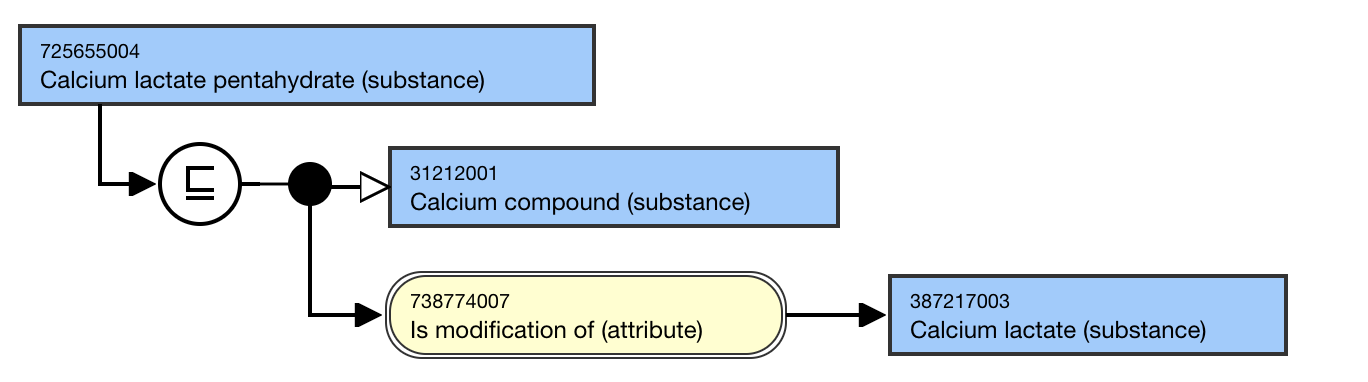
For example,
- Calcium lactate pentahydrate (substance) IS MODIFICATION OF Calcium lactate (substance)
This is the stated and inferred view.
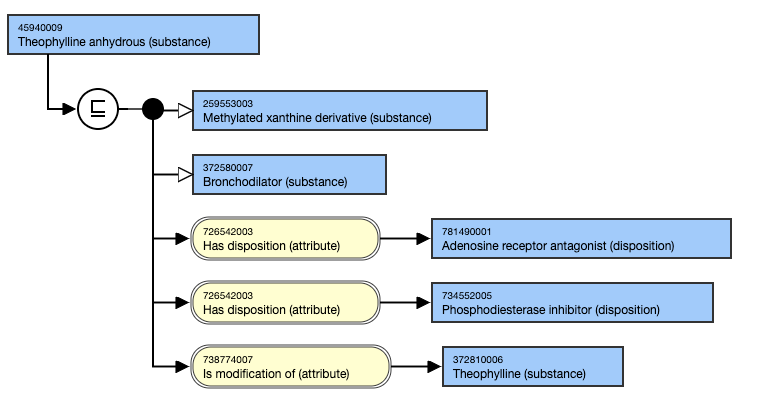
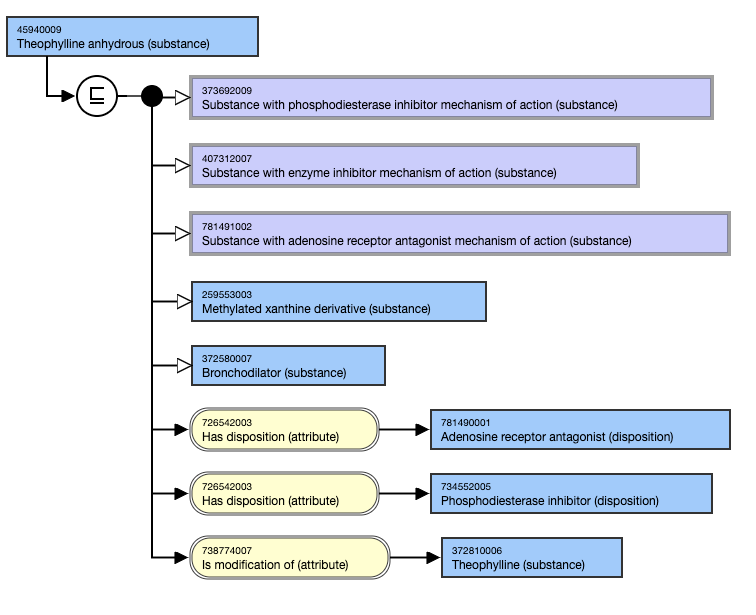
Anhydrous salts have an 738774007 | Is modification of (attribute)| relationship to the unspecified salt.
For example,
- Theophylline anhydrous (substance) IS MODIFICATION OF Theophylline (substance).
This is the stated view:
This is the inferred view:
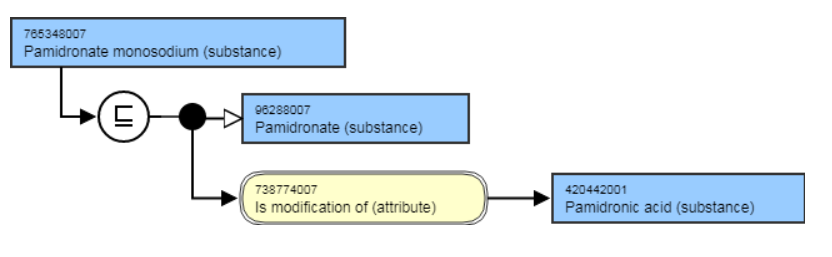
The salts are modeled with an 738774007 | Is modification of (attribute)| the acid substance.
For example,
- Pamidronate monosodium (substance) IS MODIFICATION OF Pamidronic acid (substance). This is the stated and inferred view.
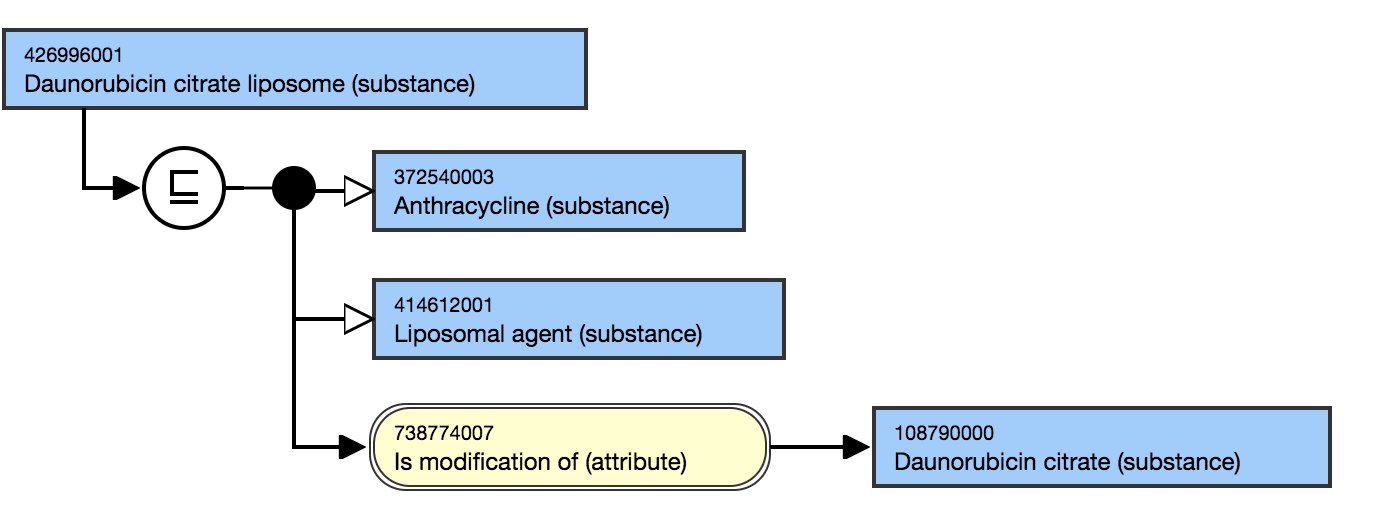
Liposomal preparations are modifications of the chemical substance.
For example,
- Daunorubicin citrate liposome (substance)|
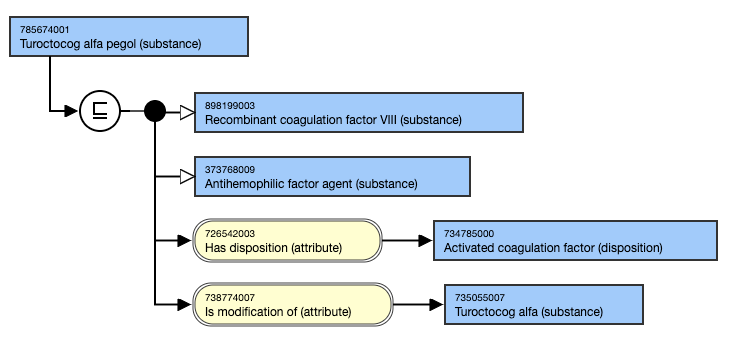
Pegylated substances should be modeled with |s modification of (attribute)| generic substance, if such a substance has been made available; not all pegylated medicinal substances have a non-pegylated form as the non-pegylated form may have been too immunogenic or too toxic.
For example,
- 785674001 |Turoctocog alfa pegol (substance)|
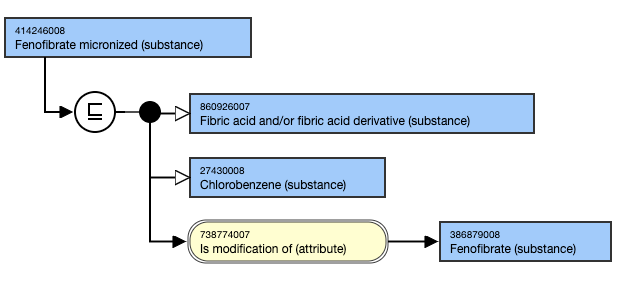
Concepts specifying a particular physical form (e.g. micronized, macrocrystal, microsphere) should have a parent concept that relates to the structure of the substance and also an Is modification of (attribute) relationship to the unspecified substance concept.
Grouper concepts should not be targets of an 738774007 | Is modification of (attribute)| relationship.
In general, an 738774007 | Is modification of (attribute)| is not applicable to prodrugs. This is because there is no requirement for the |Has active ingredient (attribute)| of medicinal products containing prodrug substances to use any relationship to the active substance to manage relationships within the medicinal product hierarchy.
For example, aspirin is not generally considered as a modification of the substance salicylic acid.
When a prodrug is an esterified form of substance, there should be an 738774007 | Is modification of (attribute)| relationship between the substance and its esterified form.
For exemplar,
- 715220007 |Tenofovir alafenamide (substance)|
- 89678001 |Cefuroxime axetil (substance)|
- 715220007 |Tenofovir alafenamide (substance)|
738774007 | Is modification of (attribute)| is not applicable:
- Between a glycan and its glycoconjugate.
- For example, 259289005 |Trimethylene glycol (substance)| is not a modification of 52086008 |Glycol (substance)|.
- To genetic engineering process variations (e.g. Somatropin(epr), Somatropin(rbe), or Somatropin(rmc) as SNOMED CT does not generally differentiate substances based on production process.
- Between a chemical element and its salt.
- For example, 387307005 |Calcium carbonate (substance)| is not a modification of |Calcium (substance)|.
Feedback