|
Page At A Glance |
Table Of Contents |
Introduction
Background
SNOMED CT terminology provides a common language that enables a consistent way of indexing, storing, retrieving, and aggregating clinical data across specialties and sites of care.
SNOMED International maintains the SNOMED CT technical design, the content architecture, the SNOMED CT content (includes the concepts table, the descriptions table, the relationships table, a history table, and ICD mappings), and related technical documentation.
Purpose
This document provides a summarized description of the content changes included in the February 2022 release of SNOMED Clinical Terms® (SCT) International Release.
It also includes technical notes detailing the known content or technical issues where the root cause is understood, the fix has been discussed and agreed to, but has yet to be implemented.
The SNOMED International Release Notes are available alongside the February 2022 International release.
Scope
This document is written for the purpose described above and is not intended to provide details of the technical specifications for SNOMED CT or encompass every change made during the release.
Audience
The audience includes National Release Centers, WHO-FIC release centers, vendors of electronic health records, terminology developers and managers who wish to have an understanding of changes that have been incorporated into the February 2022 International release.
Content Development Activity
Summary
Continuous quality improvement and enhancement of existing content is an ongoing process undertaken by SNOMED International in preparation for every release. The February 2022 International Release has seen a continuation of the work driven by contributions from: Kaiser Permanente i.e. Convergent Medical Terminology (CMT), Global Medical Device Nomenclature Agency (GMDNA), Orphanet and other domain specific collaborations as well as requests received via the Content Request System (CRS).
Additionally quality improvement activities are advanced via project driven initiatives summarized below. Additional work items impacting every release are updates to the SNOMED CT derived maps such as ICD-10 and ICD-O; details are included in these release notes.
Information about editorial decisions may be found in the SNOMED CT Editorial Guide; mapping guidance for ICD-10 can be found at this link https://confluence.ihtsdotools.org/display/DOCICD10
COVID-19
Content relating to COVID-19 can be viewed here SNOMED CT COVID-19 Related Content
Any concepts in scope for the SNOMED CT to ICD-10 mapping have been mapped and adhere to the World Health Organization current guidelines.
Quality Initiative
The Quality Initiative (QI) project is the implementation of the Quality Strategy. After a successful pilot project for the July 2018 release the next stage has been implemented for subsequent releases including February 2022.
Quality improvement tasks were deployed to improve internal structural consistency and ensure compliance with editorial policy related to the stated modeling of content. Additionally, correction or addition of defining relationships was carried out to accurately reflect current clinical knowledge and ensure the semantic reliability of descriptions associated with a concept.
Information about the project can be found here Quality Initiative - Progress (Please note, you may have to register for Confluence user account in order to access this link).
Body structure
There are approximately 35,000 anatomy concepts to be modeled by different types of 'part of' relationships. The new model will enable the automatic generation of hierarchies to further improve content quality and consistency. The integumentary system (about 2000 concepts) has been modeled. The modeling of the musculoskeletal system (about 10,000 concepts) is currently in progress. The concept model requires tooling enhancements to support nested expressions and the option of inferred relationships for transitive and reflexive attributes. We will inform the community of the schedule for the demo release when the tooling and content are ready. The demo release will help us to gather feedback to evaluate potential impact and options for future rele |
SEP and Laterality Anatomy Reference Sets
Updated and validated release file for the lateralizable body structure reference set.
Updated and validated release files for the SEP refsets.
The updates of SEP refsets and laterality refset are enhanced by new validation rules and automations as part of the authoring process.
Clinical Finding
Notice: 'Co-occurrent and due to' pattern: During the implementation of the new Description Logic features, a conflict was uncovered between the modeling of 'Co-occurrent and due to' and General Concept Inclusions (GCIs). This has resulted in the need to reconsider the modeling of "Co-occurrent and due to' and update the Editorial Guide for this area. The Editorial Guide and all concepts that are currently modeled as 'Co-occurrent and due to' will be updated over future release cycles. |
|---|
Disorder X without Disorder Y
The vast majority of existing X without Y concepts originated from ICD-9 with the specific meaning of "X disorder without mention of Y disorder". As the phraseology indicates a lack of data about disorder Y as opposed to a specific exclusion, this type of concept has not been included in ICD-10, nor proposed for ICD-11, except in the case of "Traumatic brain injury without open intracranial wound".
Addition of new X without Y concepts may only be made under the following conditions:
- The request for new content must be accompanied by a rationale as to the difference between "X disorder without Y disorder" and "X disorder."
- Approval for addition is given by the Head of Terminology.
For the most part, existing X without Y concepts will be inactivated as AMBIGUOUS with a historical MAY BE relationship to "X disorder". Exceptions to inactivation will be made on a case-by-case basis.
Notice: Inactivation reason of LIMITED/WAS_A is not allowed for any new content inactivations after the July 2018 release. The WAS_A association refset has not been updated thereafter. At the Editorial Advisory Group meeting in April 2019, agreement was reached to discontinue the maintenance of WAS_A relationships when inactivating concepts that have a historical association to an inactive concept. When changes are made to a historical relationship for a concept that was previously inactivated using WAS_A, effort will be made to assign a new historical relationship that facilitates traceability of the concept (e.g. DUPLICATE or AMBIGUOUS) as opposed to NON-CONFORMANCE TO EDITORIAL POLICY. Existing WAS_A relationships will be inactivated in a future release once a plan for batch reassignment of historical relationships has been developed. Until then, SNOMED International will not continue to use or maintain WAS_A relationships. |
|---|
Procedure
Bilateral Procedure Naming Update
Current bilateral procedure content has been updated to align with new naming conventions as documented in the Editorial Guide Bilateral Procedure Naming Conventions
Situation With Explicit Context
Revision of 'Procedure X Declined' Content
131 Situation hierarchy concepts of the pattern "Procedure X declined (situation)" have been remodeled with DECLINED for both the FSN and the Preferred Term.
Substance
For further details on the planned changes in this area, please refer to Substance Project Please note, you may have to register for Confluence user account in order to access this project and the relevant links above. |
Concepts Referencing Substance Structural Groupers of Form "X and X derivative" or "Y and Y compound"
Around 500 concepts in multiple hierarchies with FSNs that included either "X and X derivative" or "Y and Y compound" had descriptions updated to "X and/or X derivative" and "Y and/or Y compound," respectively.
Review and Update modeling for 87568004 |Hormone (substance)| and Descendants
Changes include inactivation of ambiguous groupers such as 419570005 |Hormone, synthetic hormone substitute or hormone antagonist (substance)| and 278421003 |Hormones and their metabolites and precursors (substance)| and 304360003 |Peptide hormones and their metabolites and precursors (substance)|.
Pharmaceutical/Biological Product
For further details on the planned changes in this area, please refer to the Drugs Project Please note, you may have to register for Confluence user account in order to access this project and the relevant link. |
Semi-solid Dose Form Strength Normalization
Clinical drug concepts with semi-solid dose Pharmaceutical dose forms (e.g. cream, gel, or ointment) in the International Release have strength denominators normalized to /1 gram (for w/w) or /1 milliliter (for w/v or v/v).
The Editorial Guide and content have been updated accordingly.
Lyophilized Powder Dose Form
Clinical drug concepts in the International Release should not be modeled using "lyophilized powder" Pharmaceutical dose forms. Pharmaceutical dose forms representing "lyophilized powder" will still be created in the International Release and will be available for use in a National extension. Editorial guide and existing content updated accordingly.
Products Containing Pancreatic Enzymes
Clinical drug concepts in the International Release containing pancreatic enzymes will be modeled based on the discrete enzymes; because of variability between real clinical drugs, synonyms representing a total amount in a particular product will not be included in the International Release. The editorial guide and existing content will be reviewed and updated accordingly in a future release.
Qualifier Value
Planned Inactivation of 260299005 |Number (qualifier value)| and Descendants
Following the deployment of the concrete domain functionality in SNOMED CT, concepts in the 260299005 |Number (qualifier value)| hierarchy are no longer necessary and plans have been made for their inactivation. To provide adequate time for any national extension or implementation affected by this change, concepts in the 260299005 |Number (qualifier value)| hierarchy will be inactivated in the January 2023 International Release.
Concepts will be inactivated with reason “Non-conformance to editorial policy” and no historical relationship or replacement concept will be provided.
Exceptions:
118586006 |Ratio (property) (qualifier value)| and descendants have been relocated to 118598001 |Property (qualifier value)| hierarchy.
272070003 |Ordinal number (qualifier value)| and descendants have been relocated to 362981000 |Qualifier value (qualifier value)| hierarchy.
A briefing note will also be distributed to selected Advisory and Project Groups.
Please contact info@snomed.org with any inquiries.
Physical Object
For further details on the planned changes in this area, please refer to Devices Project Please note, you may have to register for Confluence user account in order to access this project and the relevant links above. |
Compression Hosiery Class
Concepts referring to compression hosiery of a specific class (e.g. class I) have been updated to include a text definition that indicates the concept "serves as the parent of concepts representing an actual manufactured item whose description includes the explicit compression range associated with the class rating for that item."
Social concept
Person
Based on stakeholder requirements to capture the status of the donor, 2 new (person) concepts have been added as subtypes of 105457003 |Cadaver donor (person)|:
- 1187235001 |Donor after circulatory death (person)|
- 1187236000 |Donor after neurological death (person)|
Environment
Personal/Physical environment
Some subtypes of 285128009 |Personal environment (environment)| and 224777007 |Physical environment (environment)| such as 224783005 |Dirty environment (environment)| were determined to be findings about an environment. This content has been inactivated in the Environment hierarchy and add to the Clinical Finding hierarchy.
Convergent Medical Terminology (CMT)
CMT content additions:
- Injury Clinical finding concepts 360
- Mixed batch Clinical finding concept 1
- New anatomy and other concept changes to support addition of CMT content 105
Collaboration/Harmonization Agreements
Orphanet
Working in collaboration with Orphanet (http://www.orpha.net/consor/cgi-bin/index.php), creation of new concepts for the original set of prioritized rare diseases has been completed. All of the concepts added for the Orphanet project have been mapped to ICD-10.
The Production release of the SNOMED CT to Orphanet Simple Map was published in October 2021.
199 new concepts for rare diseases with a map to Orphanet entries have been added for the January 2022 International release, these new concepts will be included in the October 2022 SNOMED CT to Orphanet Simple Map release.
ICD-11 Update
A total of 503 new concepts were added for the January 2022 release.
Nutrition
Addition of ~200 nutrition concepts. Most new content is in the observable entity hierarchy (nutrition behaviors, social determinants, ability concepts), some in clinical findings (potable water access, nutrition activities of daily living findings) and a portion are regime/therapy concepts (infant feeding management).
Further information about the work of the Nutrition Care Process Clinical Project group is available here (Please note, you may have to register for Confluence user account in order to access this link).
Global Medical Device Nomenclature Agency (GMDNA)
- New concepts 28
- Concepts modified 100
- Obsolete 49
Cancer Synoptic Reporting
Over 200 concepts representing cancer synoptic reporting content were added or updated in the areas of surgical margins, tumor focality, neoplasm depth, invasion and neoplasm weight. This content primarily encompasses observable entity concepts and also includes a few supporting concepts from other hierarchies (e.g., properties, techniques).
Cancer synoptic reports are used by many member countries to record pathology examination of cancer specimens including the College of American Pathologists (US and Canada), Royal College of Pathology (UK), Royal College of Pathology Australasia (Australia, New Zealand), PALGA (The Netherlands), Swedish Society of Pathology, and others.
For more information about this project, please see Cancer Synoptic Reporting Clinical Project Group (Please note, you may have to register for Confluence user account in order to access this link).
Internal Quality Improvement
Replacement of the Stated Relationship files with the OWL Axiom refset files
A set of documentation has been developed to support the Logic Profile Enhancements. (Please note, you may have to register for Confluence user account in order to access the links below.)
Snomed OWL Toolkit -https://github.com/IHTSDO/snomed-owl-toolkit
Classifying SNOMED CT using the Snomed OWL Toolkit - https://youtu.be/-91egY9mJqA
Creating an OWL file containing SNOMED CT - https://youtu.be/sfFbMMioA_4
For any questions, please contact SNOMED International at support@snomed.org with “OWL Axiom refset files implementation question” in the subject line.
Machine Readable Concept Model (MRCM) Changes
MRCM for new attribute 1003735000 |Process acts on (attribute)
Further details can be found here MRCM changes in the January 2022 release (Please note, you may have to register for Confluence user account in order to access this project and the relevant links above).
Concrete Domains and Numeric Representation
The July 2021 International Edition was the first release after the transition to Concrete Domains.
The RF2 package is impacted as follows:
existing drug concept strength and count relationships inactivated +
existing strength and count attribute types inactivated, replaced with new ones using the same FSNs.
Inferred Relationship file changes:
Stated OWL Axiom file changes:
existing drug concept axioms updated to use concrete values +
existing strength and count attribute types inactivated, replaced with new ones using the same FSNs.
Additional Features in International Release
new separate concrete value relationship file expresses these same attributes using numeric values
new attributes used, although they will have the same FSNs as the current attributes
MRCM includes new rows to indicate that the new attribute types are expected to take a concrete domain - specifically numbers - as target values.
Please contact SNOMED International with questions at support@snomed.org with “Concrete Domains transition question” in the subject line.
SNOMED CT derived products
ICD-10 map
The SNOMED CT to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (© World Health Organization 1994) 2016 Version map (SNOMED CT to ICD-10 Map) is included in the SNOMED CT International release as a Baseline. The SNOMED CT to ICD-10 Map was created to support the epidemiological, statistical and administrative reporting needs of SNOMED International member countries and WHO Collaborating Centers.
The SNOMED CT to ICD-10 Map is released in Release Format 2 (RF2) only. It is located in the file der2_iisssccRefset_ExtendedMapFull_INT_20200731.txt, which is in the Map folder under Refset, in each of the three RF2 Release Type folders.
The SNOMED CT to ICD-10 Map is released as Refset 447562003 |ICD-10 complex map reference set (foundation metadata concept)|.
Content development activity summary
The map is a directed set of relationships from SNOMED CT source concepts to ICD-10 target classification codes. The SNOMED CT source domains for the MAP are limited to subtypes of 404684003 |clinical finding|, 272379006 |event| and 243796009 |situation with explicit context|. The target classification codes are ICD-10 2016 release.
Mapped content for January 2022
The map provided for the January 2022 release has been updated, and now represents a complete map from SNOMED CT International release to ICD-10 2016 version.
1620 newly authored concepts have been added and mapped.
The SNOMED to ICD-O (morphology) map has had a total of 102 concepts added as a result of the ICD-O 3.2 review or added due to CRS requests.
We would welcome feedback on any issues that users of the map may detect when using the map. Issues should be submitted via mapping@snomed.org
Technical Guide Exemplars
The Technical Guide Exemplars document has now been moved from the International Edition release package to a Confluence page. This page can be found as part of the ICD-10 Mapping Technical Guide (see Appendix B), which is hosted here: http://snomed.org/icd10map
SNOMED CT to OWL conversion and classification
The repository containing the toolkit enabling simple SNOMED CT to OWL conversion and classification can be found here, including documentation on its use: https://github.com/IHTSDO/snomed-owl-toolkit
Please contact SNOMED International at support@snomed.org if you would like to provide any feedback on ways to extend and improve the new toolkit.
Technical notes
Known Issues
Known Issues are content or technical issues where the root cause is understood, and the resolution has been discussed and agreed but has yet to be implemented. This can be due to a number of reasons, from lack of capacity within the current editing cycle, to the risk of impact to the stability of SNOMED CT if the fix were to be deployed at that stage in the Product lifecycle.
For the current SNOMED CT International edition, the following Known Issues were identified, and agreed to be resolved in future editing cycles:
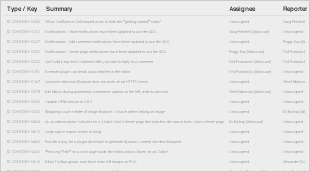
Resolved Issues
Resolved issues are Known Issues which were not fixed as part of the previous release lifecycle, but which have now been resolved in the latest release. They can also be issues found during the Alpha and Beta testing of the current release, which were resolved before the final deployment of the associated Member release. Finally they can be issues which were reported or found during the testing phase, but which have been closed without any action taken.
The Resolved Issues for the current Snomed CT International edition can be found here:

Technical updates
RF2 package format
For future reference, the RF2 package convention dictates that it contains all relevant files, regardless of whether or not there is content to be included in each particular release. Therefore, the package contains a mixture of files which contain both header rows and content data, and also files that are intentionally left blank (including only a header record). The reason that these files are not removed from the package is to draw a clear distinction between files that:
have been deprecated (and therefore removed from the package completely), due to the content no longer being relevant to RF2 in this or future releases, and
happen to contain no data in this particular release (and are therefore included in the package but left blank, with only a header record), but are still relevant to RF2, and could therefore potentially contain data in future releases.
This allows users to easily distinguish between files that have purposefully been removed or not, as otherwise if files in option 2 above were left out of the package it could be interpreted as an error, rather than an intentional lack of content in that release.
Configuration file in the RF2 package, containing Release Metadata
A new file has been included since the July 2020 International Edition, containing metadata about the Release package itself. This has been created in conjunction with feedback from the community, and as such initially contains the following fields:
effectiveTime
languageRefset(s)
humanReadableLanguageRefset(s)
licenceStatement
The file is in .JSON format, to ensure that it is both machine-readable and human-readable, and is named "release_package_information.json".
The metadata will be extended and refined going forward, in order to ensure that it contains the most useful information possible. If you have any ideas about any other useful information to include, please send them to info@snomed.org, along with a business case explaining how the information would benefit stakeholders. Please be aware that this use case will then be assessed by SNOMED International, and the new metadata will only be included in the configuration file if the business case is strong enough.
Advanced Notice of upcoming changes to the International Edition Release Schedule
As you may already know SNOMED International are transitioning to a more frequent (monthly) delivery schedule for the International Edition of SNOMED CT. The move towards more frequent releases of SNOMED CT will realise several benefits, including:
Whilst most users will continue unaffected (as they can simply continue to download the releases every 6 months as always), this transition will necessarily involve a few changes to process/packages:
NOTE: SNOMED International worked closely with Members over the past couple of years to better understand the impact of the proposed model, and have incorporated feedback into the new processes. This was designed to prevent any adverse impact to users, however if you have any further questions or concerns please contact us on support@snomed.org. |
Early visibility of impending changes in the upcoming 2022 Monthly International Edition releases
Please see the following page for details of all upcoming changes planned for 2022: https://confluence.ihtsdotools.org/display/RMT/2022+Early+Visibility+Release+Notice+-+Planned+changes+to+upcoming+SNOMED+International+Release+packages
Document linksAll links provide information that is correct and current at the time of this Release. Updated versions may be available at a later date, but if so these will need to be requested from the relevant SNOMED International teams. NOTE: To access any of the links in the pdf document, please visit the Release Notes @ https://confluence.ihtsdotools.org/display/RMT/SNOMED+CT+January+2022+International+Edition+-+SNOMED+International+Release+notes?src=contextnavpagetreemode |
Approvals
|
Draft Amendment History
|