1 Introduction
1.1 Background
SNOMED CT terminology provides a common language that enables a consistent way of indexing, storing, retrieving, and aggregating clinical data across specialties and sites of care. The SNOMED CT US Edition release is a combination of the most recent SNOMED CT International Edition and US Extension. The US Edition eliminates the need for implementers to manually combine the US Extension and International releases. SNOMED CT US Edition is the official source of SNOMED CT, which is one of a suite of standards mandated for use in US healthcare systems.
The International Health Terminology Standards Development Organisation (IHTSDO®), trading as SNOMED International (SI), maintains the SNOMED CT technical design, the content architecture, SNOMED CT content (including the concepts, descriptions, and relationships tables, history tables, and ICD mappings), and related technical documentation. SNOMED CT US Edition is developed and maintained by the United States National Library of Medicine and is available to authorized Unified Medical Language System (UMLS) Metathesaurus Licensees. This material includes SNOMED CT which is used by permission of the IHTSDO. All rights reserved.
SNOMED CT was originally created by the College of American Pathologists. "SNOMED" and "SNOMED CT" are registered trademarks of IHTSDO. You must have a UMLS Metathesaurus license to access SNOMED CT US Edition data files. License information is available from NLM (https://www.nlm.nih.gov/healthit/snomedct/snomed_licensing.html).
1.2 Purpose
This document provides a summarized description of the content changes included in the March 2019 release of the SNOMED Clinical Terms® US Edition.
It also includes technical notes detailing the known issues which have been identified. These are content or technical issues where the root cause is understood, and the fix has been discussed and agreed, but has yet to be implemented.
This document is available as a web resource as well as a PDF download alongside the March 2019 SNOMED CT US Edition and should be used in conjunction with the January 2019 SNOMED CT International Edition Release Notes.
1.3 Scope
This document is written for the purpose described above and is not intended to provide details of the technical specifications for SNOMED CT or encompass every change made during the release. These Release Notes should be used in combination with the January 2019 SNOMED CT International Edition Release Notes (https://confluence.ihtsdotools.org/display/RMT/SNOMED+CT+January+2019+International+Edition+-+SNOMED+International+Release+notes).
1.4 SNOMED CT Specification
As mentioned above, this document is not to describe the technical specifications for SNOMED CT in detail. However, briefly:
SNOMED CT is provided in UTF-8 encoded tab-delimited flat files which can be imported into any database or other software application. SNOMED CT is not software. The SNOMED CT files are designed as relational tables with each line in the file representing a row in the table. The first row of each table contains column headings. All other rows contain data.
The Concept file holds the clinical concepts that make up SNOMED CT. A concept is given meaning by its Fully Specified Name, which is held in the Description file. A concept may be distinguished from or refined by associations with other concepts using relationships, which are held in the Relationship file. Therefore, SNOMED CT data is not just a flat list of codes, but rather are data that are used as a relational database.
Unzipping the SNOMED CT US Edition release you will find the Full, Snapshot, and Delta versions. The Full release contains all of the SNOMED CT data from the beginning of SNOMED CT. It will include the full history of every single concept (when it was first introduced, if it has been edited, retired, etc.). The Snapshot version is a “snapshot” of the current SNOMED CT data. This release will provide information if the component (i.e., concept, description, relationship) is currently active or retired. The Delta release contains the new/changed content for the current release. For the US Edition the Delta will include the most recent International Edition release delta plus US Extension delta data.
For more information about SNOMED CT specification, including the information about metadata of SNOMED CT data, please see:
These documents are maintained by SNOMED International and are in reference to the International Edition. The SNOMED CT US Edition is a combination of the International and US Extensions, and thus the documentation is reliable for the US Edition as well.
2 Content Development Activity
2.1 Summary
The March 2019 SNOMED CT US Edition marks the third release with content authoring tooling and release production provided by SNOMED International Managed Service. Quality assurance of the release was completed by both NLM and SNOMED International. In October 2017, NLM sunset the United States SNOMED CT Content Request System and migrated to the SNOMED International hosted and maintained US CRS (https://us-request.ihtsdotools.org). New and modified content to the US Extension is provided by requests submitted via the US CRS. Documentation on how to submit for new or changes to content can be found on our US CRS User Guide.
In addition to 410 new concepts specific for the US, the March 2019 SNOMED CT US Edition also contains all of the new and modified content from the January 2019 SNOMED CT International Edition.
2.1.1 March 2019 US Extension of SNOMED CT Delta Statistics
The following table displays the new and inactivated concept data statistics for the 20190301 US Extension of SNOMED CT, these are the changes and additions made to the US Extension of SNOMED CT since the last release. These changes were from the approximately 685 requests for terminology inclusions and changes were submitted via US CRS (https://us-request.ihtsdotools.org) or changes for quality improvement.
Hierarchy | 20190301 US Extension New Concepts | 20190301 US Extension Inactivated Concepts |
|---|---|---|
SNOMED CT Concept (SNOMED RT+CTV3) | 410 | 64 |
Body structure (body structure) | 2 | 28 |
Clinical finding (finding) | 328 | 32 |
Environment or geographical location (environment / location) | 0 | 0 |
Event (event) | 22 | 0 |
Observable entity (observable entity) | 4 | 0 |
Organism (organism) | 0 | 2 |
Pharmaceutical / biologic product (product) | 0 | 0 |
Physical force (physical force) | 0 | 0 |
Physical object (physical object) | 0 | 0 |
Procedure (procedure) | 30 | 2 |
Qualifier value (qualifier value) | 0 | 0 |
Record artifact (record artifact) | 0 | 0 |
SNOMED CT Model Component (metadata) | 0 | 0 |
Situation with explicit context (situation) | 21 | 0 |
Social context (social concept) | 0 | 0 |
Special concept (special concept) | 0 | 0 |
Specimen (specimen) | 0 | 0 |
Staging and scales (staging scale) | 3 | 0 |
Substance (substance) | 0 | 0 |
2.1.2 March 2019 US Edition of SNOMED CT Statistics
The following table displays the data counts for SNOMED CT March 2019 US Edition (20190301 Extension + 20190131 International), January 2019 International Edition, and March 2019 US Extension.
| Release | Number of Concepts | Number of Descriptions | Number of Relationships |
|---|---|---|---|
| 20190301 US Edition of SNOMED CT | 356185 | 923583 | 2922265 |
| 20190131 International Edition of SNOMED CT | 349548 | 907283 | 2876521 |
| 20190301 US Extension of SNOMED CT | 6637 | 16300 | 45744 |
2.2 SNOMED CT derived products
2.2.1 SNOMED CT to ICD-10-CM map
The purpose of the SNOMED CT to ICD-10-CM map is to support semi-automated generation of ICD-10-CM codes from clinical data encoded in SNOMED CT for reimbursement and statistical purposes. All current pre-coordinated SNOMED CT concepts from the US Edition (US Extension + International release) within three hierarchies, (Clinical findings, Events, and Situations with Explicit Context) are potentially in scope for mapping to ICD-10-CM.
The SNOMED CT to ICD-10-CM map is released in RF2 format within the US Edition. The map is also available in a human readable format (tls_Icd10cmHumanReadableMap_US1000124_YYYYMMDD.tsv) contained within the documentation folder in the US Edition release.
The SNOMED CT to ICD-10-CM map is released as Refset 6011000124106 | ICD-10-CM extended map reference set (foundation metadata concept)|. The map data are in the file xder2_iisssccRefset_ExtendedMapSnapshot_US1000124_YYYYMMDD.txt, which is in the Map folder under Refset in the Snapshot Release Type folder.
Starting with the 2018 March SNOMED CT US Edition (20180301) release, the SNOMED CT to ICD-10-CM map refset data are now maintained as per SNOMED CT RF2 specifications. Starting with the 2018 September SNOMED CT US Edition (20180901), new 6011000124106 | ICD-10-CM complex map reference set (foundation metadata concept) data will be found in the Delta, current status data in the Snapshot, and a historical record of the refset data in the Full Release.
Additional map refset information can be found in the SNOMED CT to ICD-10-CM Release Notes (doc_Icd10cmMapReleaseNotes_Current-en-US_US1000124_YYYYMMDD.pdf) found in the Documentation folder, or online (SNOMED CT to ICD-10-CM Mapping Refset Release Notes - March 2019). Technical information (doc_Icd10cmMapTechnicalSpecifications_Current-en-US_US1000124_YYYYMMDD.pdf) for the map can also be found in the Documentation folder.
2.2.2 Route of administration
The NLM maintained Route of administration reference set (442311000124105 | Route of administration reference set) is located in the file der2_Refset_Simple(Snapshot, Delta, Full)_US1000124_YYYYMMDD.txt, which is in the Content folder under Refset, in each of the three RF2 Release Type folders. The refset contains a set of terms related to the location of administration for clinical therapeutics. The purpose of the Route of Administration refset is to define a portion of which can be used in the Drug Listing section of Structured Product Labeling (SPL), or for documentation and encoding of clinical information regarding substance administrations. Only concepts that are subtypes of the concept 284009009 | Route of administration value (qualifier value) are included in the refset. Please note the 442311000124105 | Route of administration reference set has been in static status since 2014 September US Edition release (20140901).
2.2.3 Transitive closure file
NLM provides a transitive closure script and generated transitive closure table within the SNOMED CT US Edition release. The transitive closure is the complete set of relationships between every concept and each of its super-type concepts (parents and ancestors). The transitive closure files can be used to support SQL queries when created and stored as a table in a relational database. If applied in a full release, it will contain the full history and allow for subsumption queries to be applied based on any release. The NLM transitive closure script provides a comprehensive view of all of the supertype ancestors of a concept derived by traversing all the 116680003 | is a | relationships between that concept and the root concept. The transitive closure table represents the transitive closure of the 116680003 | is a | relationships of all active concepts. NLM provides the transitive closure table in the US Edition release to provide support for easier implementation and a reference against which to check alternative algorithms.
The Transitive Closure script is located in the TransitiveClosure file within the Resources file, tls2_TranstiveClosure_US1000124_YYYYMMDD.pl. The Transitive Closure table is located in the TransitiveClosure file within the Resources file, res2_TransitiveClosure_US1000124_YYYYMMDD.txt. For the table, the superTypeId is the id of the concept playing the supertype role, set to an Identifier of a concept. The subTypeId - Id of the concept playing the subtype role. Set to an Identifier of a concept.
3 Technical Notes
 3.1 Known Issues
3.1 Known Issues
Known Issues are content or technical issues where the root cause is understood, and the resolution has been discussed and agreed but has yet to be implemented. This can be due to a number of reasons, from lack of capacity within the current editing cycle, to the risk of impact to the stability of SNOMED CT if the fix were to be deployed at that stage in the Product lifecycle. In addition to the known issues reported by the IHTSDO for SNOMED CT International Edition (SNOMED CT January 2019 International Edition - SNOMED International Release notes), the following are known issues for the SNOMED CT US Edition.
For the latest version of the SNOMED CT Managed Service - US Edition, the following Known Issues were identified, and agreed to be resolved in the next editing cycle:
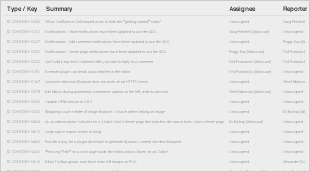
3.2 Resolved Issues
Resolved issues are Known Issues which were not fixed as part of the previous release lifecycle, but which have now been resolved for this latest US Edition. These can be issues found during the Pre-Production or Production phases of the testing of the current release, which were resolved before the final deployment of the final production release. Finally these can be issues which were reported or found during the testing phase, but which have been closed without any action taken.
In addition to the Resolved Issues reported by the IHTSDO for SNOMED CT International Edition (SNOMED CT January 2019 International Edition - SNOMED CT Release Notes), the following Resolved Issues for the latest SNOMED CT US Edition:

3.3 Technical Updates
In addition to the Technical Updates reported in the SNOMED CT January 2019 International Edition - SNOMED International Release notes, the following technical updates pertain to the US Edition:
3.2.1 File Changes Expected in the September 2019 US Edition
To continue to align with the SNOMED CT International Edition, the the following changes will be implemented starting with the September 2019 US Edition. These changes will allow for the continued efforts towards having a zip file including only data with additional supplemental documentation and artifacts available separately.
- Deprecation of the Documentation folder
- This aligns with the SNOMED CT International Edition release package which no longer includes a Documentation file.
- Deprecation of the doc_Icd10MapTechnicalGuideExemplars_[date].xlsx file
- This follows the decision by SNOMED International, first announced with the July 2018 International Edition (see 3.3.4 - Deprecation of the Technical Guide Exemplars document from the International Edition package). Going forward the file will be available from SNOMED International (http://snomed.org/icd10map).
- Removal of SNOMED CT to ICD-10-CM documentation and resources from the US Edition release zip file
- The SNOMED CT to ICD-10-CM documentation and resources will be made available alongside the US Edition of SNOMED CT as a bundled download - SNOMED_CT_to_ICD-10-CM_Resources_YYYYMMDD.zip. The following resources will be updated and bundled together and made available with each new US Edition release:
- doc_Icd10cmMapReleaseNotes_Current-en-US_US1000124_YYYYMMDD.pdf (will be available as an online resources and in the download)
- doc_Icd10cmMapTechnicalGuideExemplars_Current-en-US_US1000124_YYYYMMDD.xlsx
- doc_Icd10cmMapTechnicalSpecifications_Current-en-US_US1000124_YYYYMMDD.pdf (will be available as an online resource and in the download)
- tls_Icd10cmHumanReadableMap_US1000124_YYYYMMDD.tsv
- The SNOMED CT to ICD-10-CM documentation and resources will be made available alongside the US Edition of SNOMED CT as a bundled download - SNOMED_CT_to_ICD-10-CM_Resources_YYYYMMDD.zip. The following resources will be updated and bundled together and made available with each new US Edition release:
- Deprecation of the Documentation folder
Any questions or comments regarding these upcoming changes can be sent to NLM Customer Support https://support.nlm.nih.gov/.
3.2.2 Combination of two new refsets - OWLAxiom and OWLOntology
The US Edition is following the International Edition with the changes to the Stated Relationships and two new OWL refsets.
Please see section "2.3.1 Logic Profile Enhancements" in the January 2019 SNOMED CT International Edition Release Notes for full details of the reason behind the introduction of these new refsets. After the September 2018 Demonstration release, it was agreed to combine these two files into one "OWL Expression" file, which now contains both OWL Axiom + OWL Ontology refsets in the same file, in each section of the International Release package (Full, Snapshot and Delta):
- sct2_sRefset_OWLExpressionFull_INT_20190131.txt
- sct2_sRefset_OWLExpressionSnapshot_INT_20190131.txt
- sct2_sRefset_OWLExpressionDelta_INT_20190131.txt
The decision was taken to place the files in the "Terminology" folder in the release package (as opposed to the "Refset" folder), because these files are designed to eventually replace the Stated relationship files (in July 2019), and therefore contain core content which needs to be included in the Terminology folder.
3.2.3 IMPORTANT CHANGES in the September 2019 US Edition
Replacement of the Stated Relationship files with the new OWL Axiom refset files
In the September 2019 release:
- The International Edition and US Extension (therefore the US Edition) packages will include both inactive Stated Relationships plus a complete OWL file (combined) from July 2019 (International content) and September 2019 (US Extension content) effectiveTime onwards (ie) with NO history of OWL records that weren’t included in the January 2019 International Edition and March 2019 US Edition package (as opposed to the optional package).
- A separate optional package containing an OWL (combined) Delta file, identifying which of the concepts have had modelling changes in relation to the optional package detailed in section 3.3.3 of the International Release notes ("Optional "on demand" package in addition to the January 2019 International edition").
- No support for Stated Relationships will be provided from this point onwards, however we will continue to include the inactivated Stated Relationships in future International Edition packages, until it is decided that this is no longer required.
- Anyone who isn’t yet ready to move forward with OWL will therefore remain on the March 2019 US Edition until they are ready to update to OWL with the September 2019 version onwards.
- The inferred relationship file will maintain the same format and structure, though it will no longer contain all necessary and sufficient conditions. The inferred relationship file is represented in Necessary Normal Form for distribution of relationships. It is a collection of all the necessary conditions and represents a subset of the full semantics from the 2018 July release and onwards. Most users will benefit from the improvements in the inferred relationships without requiring changes to their existing systems.
A set of documentations has been developed to support the Logic Profile Enhancements.
- SNOMED DL Profile Enhancements - https://docs.google.com/document/d/1tqNEA6S4fEF4fgj15OPabYA2E0VTz8epxvRRwczKizQ/edit?usp=sharing
- SNOMED CT Logic Profile Specification - http://snomed.org/lps
- SNOMED CT OWL Guide (OWL Refsets specification) - http://snomed.org/owl
- Necessary Normal Form for Inferred Relationships - https://docs.google.com/document/d/1dt0r0aetwpwmHOfiT9wt0EVukVLRvVjXYUn_vq-QhIM/edit?usp=sharing
- Snomed OWL Toolkit - https://github.com/IHTSDO/snomed-owl-toolkit
- Classifying SNOMED CT using the Snomed OWL Toolkit - https://youtu.be/-91egY9mJqA
- Creating an OWL file containing SNOMED CT - https://youtu.be/sfFbMMioA_4
3.2.4 RF2 package format
Similar to the International Edition, the US Edition will follow the technical specifications for RF2 packaging format.
For future reference, the RF2 package convention dictates that it contains all relevant files, regardless of whether or not there is content to be included in each particular release. Therefore, the package contains a mixture of files which contain both header rows and content data, and also files that are intentionally left blank (including only a header record). The reason that these files are not removed from the package is to draw a clear distinction between
1 ...files that have been deprecated (and therefore removed from the package completely), due to the content no longer being relevant to RF2 in this or future releases, and
2. ...files that just happen to contain no data in this particular release (and are therefore included in the package but left blank, with only a header record), but are still relevant to RF2, and could therefore potentially contain data in future releases.
This allows users to easily distinguish between files that have purposefully been removed or not, as otherwise if files in option 2 above were left out of the package it could be interpreted as an error, rather than an intentional lack of content in that release.
3.4 Content and Technical Reminders
3.4.1 LIMITED/WAS A association refset inactivation
Reminder
Inactivation reason of LIMITED/WAS A is not allowed for any content after the July 2018 release. The WAS A association refset has not been updated thereafter.
Background
In 2015, a proposal was made to inactivate 159083000 |WAS A (attribute)| relationship and stop updating the 900000000000528000|WAS A association reference set (foundation metadata concept)| at the Editorial Advisory Group.
Since these recommendations were approved, a formal proposal for the technical approach to batch updating the terminology was created and a notice of the proposed inactivation sent to the Community of Practice.
The implementation of changes was postponed following feedback on utility for implementation and the potential impact to customers who were still using RF1.
The matter was discussed again at the meeting of the Editorial Advisory Group in Bratislava in October 2017. Since the requirements and potential issues can be addressed by deriving such information from the RF2 release format, the recommendation is to proceed with the decision for implementation after the July 2018 release.
3.5 Feedback and suggestions
We welcome questions, comments or suggestions to improve the quality, accuracy and usability of the SNOMED CT US Edition. Please send feedback to NLM Customer Support https://support.nlm.nih.gov/
Approvals
|
Draft Amendment History
|
