Approvals
Download .pdf here: |
Draft Amendment History
|
Introduction
SNOMED CT terminology provides a common language that enables a consistent way of indexing, storing, retrieving, and aggregating clinical data across specialties and sites of care.
The International Health Terminology Standards Development organisation (IHTSDO®), trading as SNOMED International, maintains the SNOMED CT technical design, the content architecture, the SNOMED CT content (includes the concepts table, the descriptions table, the relationships table, a history table, and ICD mappings), and related technical documentation.
The SNOMED CT Irish Extension - packages these components for release every April and October, to be used with the latest SNOMED CT International Edition release.
The same content can be viewed online using the SNOMED CT browser, and eHealth Ireland website
Background
This document provides a summarized description of the content changes included in the April 2022 Production release of the SNOMED Clinical Terms® Managed Service Ireland Extension package.
It will also include technical notes detailing the known issues which have been identified (should any of these exist). These are content or technical issues where the root cause is understood, and the fix has been discussed and agreed, but has yet to be implemented.
This Ireland Extension package is dependent upon, and should therefore be consumed in conjunction with the SNOMED CT® January 2022 International Edition release.
Scope
This document is written for the purpose described above and is not intended to provide details of the technical specifications for SNOMED CT or encompass every change made during the release.
Content Development Activity
Summary
Content from the SNOMED CT® January 2022 International Edition release has been included, alongside additional components for use in Ireland.
This extension contains concepts, relationships and reference sets for healthcare professionals.
New and Updated Content
New content in this release:
This is the 7th SNOMED CT Irish Edition Production release.
New and updated content
Concept statistics
File | Changes |
Concept | 35 records added/updated + 1 records inactivated |
Description (EN) | 87 records added/updated + 0 records inactivated |
Language (EN) | 3157 records added/updated + 8776 records inactivated |
Inferred Relationship | 67 records added/updated + 2 records inactivated |
OWL Expression | 35 records added/updated + 1 records inactivated |
TextDefinition | 0 records added/updated |
Association Reference | 1 records added/updated + 0 record inactivated |
AttributeValue | 4 records added/updated + 0 record inactivated |
RefsetDescriptor | 7 records added (for new refsets this cycle) |
The SNOMED Irish National Release Centre published
Table 1.1 New concepts in the Irish Edition April release
Total number of concepts | 35 |
Assessment scales | 3 |
| Disorder | 1 |
| Finding | 1 |
| Foundation metadata concept | 1 |
| Medicinal Product | 1 |
| Observable entity | 1 |
| Procedure | 7 |
| Record artifact | 8 |
| Regime/therapy | 3 |
| Situation | 2 |
| Supplier | 1 |
Table 1.2
Relationships | |
|---|---|
| Total active relationships | 87 |
| Inactivation's | 0 |
| Total active descriptions | 87 |
Refer to Appendix 1, Table 1.2 to view the new relationships
This content was derived through the Dataset Specification Process (DSMP )and includes concepts from
Table 1.3
Reference sets
Chronic Disease Management Refset | 0 records added/updated |
Coronavirus Refset | 0 records added/updated |
Gynaecology Discharge Summary Refset | 0 records added/updated |
Irish National Early Warning Score Refset | 0 records added/updated |
Make Every Contact Count Ireland refset | 0 records added/updated |
Safeguarding Ireland refset | 0 records added/updated |
eServices Ireland refset | 0 new record added/updated |
Ireland Nursing & Midwifery Quality | 1 new record added, 1 updated + 1 record inactivated |
Antimicrobial stewardship Performance Indicator Measurement System (APIMS) Data Set for Ireland | 202 new records for this brand new refset |
Dentistry Refset Ireland | 667 new records for this brand new refset |
Lymphodema Ireland Refset | 17 new records for this brand new refset |
National Ambulance Service Ireland Refset | 111 new records for this brand new refset |
Patient Flow Datset Ireland Refset | 247 new records for this brand new refset |
Public Health Nurse clinical caseload dataset Ireland refset | 91 new records for this brand new refset |
Table 1.4
Text Definitions
| Text definitions (metadata) | 3 |
Table 1.5
Content Promoted through CRS from IPU and acute medicines management programme
| Sept content 1/10/2021 | 2 |
| November content 12/11/2021 | 33 |
| January content 9/2/2022 | 15 |
| March content 29/3/2022 | 35 |
| Total since Oct 2021 release: | 103 |
Table 1.6
Content Promoted from other extensions to SNOMED International
| Content promoted from other extensions | 3 |
The SNOMED Irish National Release Centre promoted 103 new concepts and these will be available in the SNOMED CT May 2022 International Edition release.
Refer to Appendix 2, Table 1.2 to view
Technical notes
Known Issues
Known Issues are content or technical issues where the root cause is understood, and the resolution has been discussed and agreed but has yet to be implemented. This can be due to a number of reasons, from lack of capacity within the current editing cycle, to the risk of impact to the stability of SNOMED CT if the fix were to be deployed at that stage in the Product lifecycle.
For the SNOMED CT Managed Service - Ireland Extension Release, the following Known Issues were identified, and agreed to be resolved in the next editing cycle:
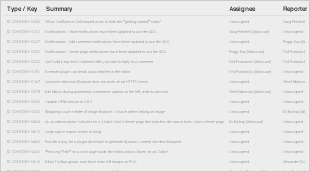
Resolved Issues
Resolved Issues are those where the resolution has been discussed and agreed, and has been implemented in time for this release.
For the SNOMED CT Managed Service - Ireland Extension Release, the following Issues were Resolved:

Notice of changes to the International Edition Release Schedule
As you may already know SNOMED International have transitioned to a monthly delivery schedule for the International Edition of SNOMED CT. The move towards more frequent releases of SNOMED CT will realize several benefits, including:
- The potential to be able to get content changes into the terminology in a shorter time frame.
- The fostering of better interoperability, as a result of entities being able to consume release content that is more aligned with other organizations.
- The prevention of circular dependencies that occur in longer projects, due to the move towards smaller, more manageable authoring projects.
- More automated validation services, as a result of the inherent removal of the Alpha/Beta stages in the Release cycle.
Whilst most users will continue unaffected (as they can simply continue to download the releases every 6 months as always), this transition will necessarily involve a few changes to process/packages:
- Delta files have been removed from both International and Managed Service release packages, including the Ireland Extension. A Delta Generation service will be provided for those who need it. The Delta Generation Tool allows users to create their own Delta between two fixed release dates - you can find it here:
- The ICD-O/ICD-10 Maps will continue to be published in each Monthly International Edition release package (in line with that month's content) for the foreseeable future, unless we experience issues with the new process in Production, and they need to be removed at a later date.
The first monthly Release of the SNOMED CT International Edition was published on the 28th February 2022 with the Delta files having been removed, and therefore they will be removed from the Ireland Extension from the April 2022 Release onwards.
Please note - While the SNOMED CT International Edition is moving to monthly releases, the Ireland Extension of SNOMED CT will remain on the current bi-annual release schedule of April and October for 2022.
Appendix 1
Table 1.1 New concepts
| 11621000220104 | Irish Maternity Early Warning System (assessment scale) | (assessment scale) |
| 84741000220100 | Irish Paediatric Early Warning System (assessment scale) | (assessment scale) |
| 84901000220104 | Infective exacerbation of cystic fibrosis (disorder) | (disorder) |
| 74901000220105 | Columnar cell lesion of breast (finding) | (finding) |
| 74921000220101 | Antimicrobial stewardship Performance Indicator Measurement System (APIMS)Data Set for Ireland (foundation metadata concept) | (foundation metadata concept) |
| 41661000220104 | Breastcheck screening service reference set (foundation metadata concept) | (foundation metadata concept) |
| 74931000220103 | Dentistry refset Ireland (foundation metadata concept) | (foundation metadata concept) |
| 84731000220109 | Lymphodema reference set Ireland (foundation metadata concept) | (foundation metadata concept) |
| 61681000220104 | National ambulance service reference set (foundation metadata concept) | (foundation metadata concept) |
| 73041000220108 | Patient Flow Dataset Ireland (foundation metadata concept) | (foundation metadata concept) |
| 61671000220102 | Public health nurse clinical caseload dataset Ireland (foundation metadata concept) | (foundation metadata concept) |
| 62051000220105 | Janssen COVID-19 Vaccine 0.5 millilitre suspension injection Janssen Inc (medicinal product) | (medicinal product) |
| 62071000220101 | Personal public service number (observable entity) | (observable entity) |
| 84881000220101 | Bed rail risk assessment (procedure) | (procedure) |
| 11661000220109 | Care bundle (procedure) | (procedure) |
| 11671000220103 | Care bundle for medical device (procedure) | (procedure) |
| 84761000220101 | Face, legs, activity, cry, consolability pain assessment scale (procedure) | (procedure) |
| 11651000220107 | Introduction situation, background assessment recommendation (procedure) | (procedure) |
| 84871000220104 | Manual handling risk assessment (procedure) | (procedure) |
| 84891000220103 | Subcutaneous cannulation (procedure) | (procedure) |
| 84821000220100 | Frailty care plan (record artifact) | (record artifact) |
| 84861000220105 | Home support service care plan (record artifact) | (record artifact) |
| 84791000220108 | Incontinence associated dermatitis care plan (record artifact) | (record artifact) |
| 84841000220106 | Neurogenic bowel care plan (record artifact) | (record artifact) |
| 84781000220105 | Pressure ulcer prevention care plan (record artifact) | (record artifact) |
| 84851000220108 | Urinary catheter care plan (record artifact) | (record artifact) |
| 84951000220100 | Urinary incontinence care plan (record artifact) | (record artifact) |
| 84831000220102 | Wound care plan (record artifact) | (record artifact) |
| 84801000220109 | Care of incontinence sheath (regime/therapy) | (regime/therapy) |
| 84961000220103 | Care of neurogenic bowel (regime/therapy) | (regime/therapy) |
| 84811000220107 | Care of postnatal depression (regime/therapy) | (regime/therapy) |
| 11631000220101 | Cardiotochogram indicated (situation) | (situation) |
| 11641000220105 | Postnatal risk factors (situation) | (situation) |
| 62041000220108 | Janssen Inc. (supplier) | (supplier) |
Appendix 2
Table 1.2
These are new incoming relationships.
| 273249006 | Assessment scales (assessment scale) | (assessment scale) |
| 76752008 | Breast structure (body structure) | (body structure) |
| 361713003 | Entire subcutaneous tissue (body structure) | (body structure) |
| 321667001 | Respiratory tract structure (body structure) | (body structure) |
| 195647007 | Acute respiratory infection (disorder) | (disorder) |
| 17097001 | Chronic disease of respiratory system (disorder) | (disorder) |
| 177010002 | Chronic infectious disease (disorder) | (disorder) |
| 116339002 | Breast finding (finding) | (finding) |
| 86569001 | Postpartum state (finding) | (finding) |
| 446609009 | Simple type reference set (foundation metadata concept) | (foundation metadata concept) |
| 787859002 | Vaccine product (medicinal product) | (medicinal product) |
| 55465005 | Columnar cell atypia (morphologic abnormality) | (morphologic abnormality) |
| 6920004 | Defect (morphologic abnormality) | (morphologic abnormality) |
| 423901009 | Identification code (observable entity) | (observable entity) |
| 410604004 | Subject of record (person) | (person) |
| 337636000 | Incontinence sheath (physical object) | (physical object) |
| 258620006 | Vascular cannula (physical object) | (physical object) |
| 223490009 | Appliance procedures (procedure) | (procedure) |
| 445536008 | Assessment using assessment scale (procedure) | (procedure) |
| 710955000 | Biomedical equipment procedure (procedure) | (procedure) |
| 42825003 | Cannulation (procedure) | (procedure) |
| 11661000220109 | Care bundle (procedure) | (procedure) |
| 225297008 | Care planning and problem solving actions (procedure) | (procedure) |
| 9718006 | Polymerase chain reaction analysis (procedure) | (procedure) |
| 399150003 | Polymerase chain reaction test for severe acute respiratory syndrome (procedure) | (procedure) |
| 118726005 | Procedure on subcutaneous tissue (procedure) | (procedure) |
| 408767007 | Procedure with a clinical finding focus (procedure) | (procedure) |
| 408766003 | Procedure with a procedure focus (procedure) | (procedure) |
| 225338004 | Risk assessment (procedure) | (procedure) |
| 416325004 | Vascular cannula procedure (procedure) | (procedure) |
| 255212004 | Acute-on-chronic (qualifier value) | (qualifier value) |
| 410512000 | Current or specified time (qualifier value) | (qualifier value) |
| 129265001 | Evaluation - action (qualifier value) | (qualifier value) |
| 410535002 | Indicated (qualifier value) | (qualifier value) |
| 441862004 | Infectious process (qualifier value) | (qualifier value) |
| 257867005 | Insertion - action (qualifier value) | (qualifier value) |
| 129271007 | Management - action (qualifier value) | (qualifier value) |
| 734163000 | Care plan (record artifact) | (record artifact) |
| 387672006 | Cardiotochogram (regime/therapy) | (regime/therapy) |
| 309637002 | Care of equipment categorized by device (regime/therapy) | (regime/therapy) |
| 225365006 | Care regime (regime/therapy) | (regime/therapy) |
| 133877004 | Therapeutic regimen (regime/therapy) | (regime/therapy) |
| 225219005 | Verbal communication interventions (regime/therapy) | (regime/therapy) |
| 318331000221102 | Active immunity stimulant role (role) | (role) |
| 708538000 | Procedure indicated (situation) | (situation) |
| 243796009 | Situation with explicit context (situation) | (situation) |
| 774164004 | Supplier (supplier) | (supplier) |
Table 1.4
Text definitions
| Id | FSN | SemTag | Lang | Term |
| 84741000220100 | Irish Paediatric Early Warning System (assessment scale) | (assessment scale) | en | Irish Paediatric early warning system (child (< 16 years) in-patient in the acute setting) IPEWS is a system which involves the anticipation, recognition, escalation, response and evaluation of the management of clinical deterioration |
| 11661000220109 | Care bundle (procedure) | (procedure) | en | A care bundle is a collection of interventions that may be applied to the management of a particular condition. |
| 11651000220107 | Introduction situation, background assessment recommendation (procedure) | (procedure) | en | ISBAR (Introduction, Situation, Background Assessment, Recommendation) is such a tool. ISBAR organises a conversation into the essential elements in the transfer of information from one source to another. |
Table 1.5
| Product containing precisely belatacept 250 mg powder for concentrate for solution for infusion (clinical drug) |
| Product containing precisely clarithromycin (as clarithromycin lactobionate) 500 mg/ 1vial powder for concentrate solution for infusion |
| Product containing precisely dacarbazine (as dacarbazine citrate) 1000 mg powder for conventional release solution for infusion (clinical drug) |
| Product containing precisely dacarbazine (as dacarbazine citrate) 500 mg powder for conventional release solution for infusion (clinical drug) |
| Product containing precisely decitabine 50 mg/1 vial powder for concentrate for solution for infusion (clinical drug) |
| Product containing precisely dexrazoxane (as dexrazoxane hydrochloride) 500 mg/ 1 vial powder for concentrate for solution for infusion |
| Product containing precisely efmoroctocog alfa 1000 units/ 1 vial powder for solution for injection (clinical drug) |
| Product containing precisely efmoroctocog alfa 1500 units/ 1 vial powder for solution for injection (clinical drug) |
| Product containing precisely efmoroctocog alfa 2000 units/ 1 vial powder for solution for injection (clinical drug) |
| Product containing precisely efmoroctocog alfa 250 units/ 1 vial powder for solution for injection (clinical drug) |
| Product containing precisely efmoroctocog alfa 3000 units/ 1 vial powder for solution for injection (clinical drug) |
| Product containing precisely efmoroctocog alfa 500 units/ 1 vial powder for solution for injection (clinical drug) |
| Product containing precisely epoprostenol (as epoprostenol sodium) 0.5 mg/1 vial powder for conventional release solution for infusion |
| Product containing precisely epoprostenol (as epoprostenol sodium) 1.5 mg/1 vial powder for conventional release solution for infusion |
| Product containing precisely esomeprazole (as esomeprazole sodium) 40 mg/1 vial powder for conventional release solution for injection |
| Product containing precisely etanercept 10 mg/1 vial powder for conventional release solution for injection (clinical drug) |
| Patisiran sodium (substance) |
| Product containing only alemtuzumab in parenteral dose form (medicinal product form) |
| Product containing only clofarabine in parenteral dose form (medicinal product form) |
| Product containing precisely alemtuzumab 10 milligram/1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely clofarabine 1 milligram/1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely mitoxantrone (as mitoxantrone hydrochloride) 2 milligram/1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing only mitoxantrone in parenteral dose form (medicinal product form) |
| Product containing precisely botulinum toxin type A 100 units powder for conventional release solution for injection (clinical drug) |
| Product containing precisely botulinum toxin type A 50 units powder for conventional release solution for injection (clinical drug) |
| Product containing precisely botulinum toxin type A 125 units powder for conventional release solution for injection (clinical drug) |
| Product containing precisely carfilzomib 10 mg powder for solution for infusion (clinical drug) |
| Product containing precisely carfilzomib 30 mg powder for solution for infusion (clinical drug) |
| Product containing precisely carfilzomib 60 mg powder for solution for infusion (clinical drug) |
| Product containing precisely cefuroxime (as cefuroxime sodium) 50 mg/1 each powder for conventional release solution for injection |
| Product containing precisely migalastat (as migalastat hydrochloride) 123 mg/1 each conventional release oral capsule |
| Product containing precisely niraparib (as niraparib tosilate monohydrate) 100 mg/1 each conventional release oral capsule |
| Product containing precisely talazoparib (as talazoparib tosylate) 250 mcg/1 each conventional release oral capsule |
| Product containing precisely talazoparib (as talazoparib tosylate) 1mg/1 each conventional release oral capsule |
| Product containing precisely dexamethasone phosphate (as dexamethasone sodium phosphate) 1 mg/1 ml conventional release eye drops, solution |
| Product containing precisely hydrocortisone sodium phosphate 3.35 mg/1 ml conventional release eye drops, solution (clinical drug) |
| Product containing precisely proxymetacaine hydrochloride 5 mg/1ml conventional release eye drops, solution (clinical drug) |
| Product containing precisely apraclonidine (as apraclonidine hydrochloride) 10 mg/1 ml conventional release eye drops, solution |
| Product containing precisely bromfenac (as bromfenac sodium) 900 mcg/1 ml conventional release eye drops, solution |
| Product containing precisely carmellose sodium 10 mg/1 ml conventional release eye drops, solution (clinical drug) |
| Product containing precisely carmellose sodium 5 mg/1 ml conventional release solution for eye drops (clinical drug) |
| Product containing precisely diclofenac sodium 1 mg/1 ml conventional release solution for eye drops (clinical drug) |
| Product containing precisely nepafenac 1 mg/1 ml conventional release suspension for eye drops (clinical drug) |
| Product containing precisely ketorolac trometamol 5 mg/1 ml conventional release solution for eye drops (clinical drug) |
| Product containing precisely hypromellose 3.2 mg/1 ml conventional release solution for eye drops (clinical drug) |
| Product containing precisely hexylresorcinol 2.4 mg/1 each lozenge (clinical drug) |
| Product containing precisely aciclovir (as aciclovir sodium) 250 mg/1 vial powder for conventional release solution for infusion |
| Product containing precisely alglucosidase alfa 50 mg/1 vial powder for concentrate for conventional release solution for infusion (clinical drug) |
| Product containing precisely aminolevulinic acid hydrochloride (as 5-aminolevulinic acid) 30 mg/1 ml powder for conventional release oral solution ) |
| Product containing precisely anidulafungin 100 mg/1 vial powder for concentrate for solution for infusion (clinical drug) |
| Product containing precisely antithymocyte immunoglobulin rabbit 25 mg/1 each powder for conventional release solution for infusion (clinical drug) |
| Product containing precisely bendamustine hydrochloride 25 mg powder for concentrate for solution for infusion. (clinical drug) |
| Product containing precisely blinatumomab 38.5 mcg powder for concentrate for solution for infusion (clinical drug) |
| Product containing precisely ammonia 350mg/1g conventional release cutaneous emulsion (clinical drug) |
| Product containing precisely betamethasone (as betamethasone valerate) 1 mg/1g cutaneous foam |
| Product containing precisely paclitaxel (as albumin bound paclitaxel) 5 mg/1 ml powder for dispersion for infusion (clinical drug) |
| Product containing precisely colistimethate sodium 1 MIU/1 vial powder for solution for injection/infusion (clinical drug) |
| Product containing precisely colistimethate sodium 2 MIU/1 vial powder for solution for injection/infusion (clinical drug) |
| Product containing precisely cetuximab 5 milligram/1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely fludarabine phosphate 25 milligram/1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely gemcitabine (as gemcitabine hydrochloride) 100 milligram /1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely gemcitabine (as gemcitabine hydrochloride) 38 milligram /1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely gemcitabine (as gemcitabine hydrochloride) 40 milligram /1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely obinutuzumab 25 milligram/1 milliliter conventional release solution for infusion (clinical drug) |
| Product containing precisely patisiran (as patisiran sodium) 2 milligram/1 milliliter conventional release solution for infusion (clinical drug) |
Table 1.6
Content promoted from other extensions to International
1106021000000101 |Insertion of magnetic marker into breast using stereotactic mammography guidance (procedure)| has ben added to the International release. This response is subject to change until the time of release. |
1111791000000108 |Insertion of magnetic marker into breast using X-ray guidance (procedure)| has been added to the International release. This response is subject to change until the time of release. |
1106671000000108 |Insertion of magnetic marker into breast using ultrasonographic guidance (procedure)| has been added to the International release. This response is subject to change until the time of release. |
