Introduction

The Ministry of Health works with health and disability agencies and their industry partners to develop SNOMED CT® reference sets, maps, language reference sets and other content for digital health solutions in New Zealand.
The SNOMED CT New Zealand Extension - SNOMED NZ Extension, for short - packages these components for release every April and October, to be used with the latest SNOMED CT International Edition release.
The same content can be viewed online using the SNOMED CT browser and accessed via our SNOMED NZ Snowstorm terminology service API at https://snomednz.digital.health.nz/
We welcome your feedback and participation. For more information about SNOMED CT implementation in New Zealand, see the SNOMED NZ pages on our website or follow SNOMED NZ topics on our news site
Background
This document provides a summarized description of the content changes included in the October 2021 production release of the SNOMED NZ Extension package.
It will also include technical notes detailing any known issues which have been identified. These are content or technical issues where the root cause is understood, and the fix has been discussed and agreed, but has yet to be implemented.
This SNOMED NZ Extension package is dependent upon, and should therefore be consumed in conjunction with the SNOMED CT July 2021 International Edition release.
Content development activity
Summary
Content from the SNOMED CT July 2021 International Edition release has been incremented, using additional components for use in New Zealand.
The October 2021 release includes updates to the previously released content in the SNOMED NZ Extension release and introduces some new content.
New and updated content
Concept statistics
The SNOMED NZ National Release Centre promoted 13 new concepts into the SNOMED CT July 2021 International Edition release.
Table 1. New concepts in the SNOMED International July 2021 release by hierarchy
| Total number of concepts | 13 |
|---|---|
| Clinical finding (finding) | 5 |
| Procedure (procedure) | 1 |
| Qualifier value (qualifier value) | 1 |
| Staging and scales (staging scale) | 6 |
Refer to Appendix 1, Table 1.1 to view the 13 new concepts.
There are 250 new concepts in this release.
Table 2. New concepts in the SNOMED NZ Extension October 2021 release by hierarchy
Total number of concepts | 250 |
|---|---|
Clinical finding (finding) | 8 |
Observable entity (observable entity) | 8 |
Procedure (procedure) | 87 |
| Qualifier value (qualifer value) | 24 |
| Situation with explicit context (situation) | 86 |
SNOMED CT Model Component (metadata) | 25 |
| Social context (social concept) | 11 |
Specimen (specimen) | 1 |
Refer to Appendix 1, Table 2.1 to view the 250 new concepts.
Description statistics
The SNOMED NZ NRC promoted 1 new description to the SNOMED International Edition July 2021 release.
Table 3. New description in the SNOMED International July 2021 release
SNOMED CT Concept ID (SCTID) | SNOMED CT Fully Specified Name (FSN) | New description |
|---|---|---|
446974000 | Body mass index centile (observable entity) | BMI (body mass index) centile |
There are 7 new descriptions in this release.
Table 4 New descriptions in the SNOMED NZ Extension October 2021 release
| SNOMED CT Concept ID (SCTID) | SNOMED CT Fully Specified Name (FSN) | New description |
|---|---|---|
| 310139001 | Breast surgery service (qualifier value) | General surgery - breast |
| 609520005 | Disorder of fetal structure (disorder) | Fetal structure abnormality |
| 1137688001 | Ex-electronic cigarette user for less than one year (finding) | Ex-vaping for less than 1 year |
| 1137692008 | Ex-electronic cigarette user for more than one year (finding) | Ex-vaping for more than 1 year |
| 310151004 | Gastrointestinal surgery service (qualifier value) | Gastroenterology surgical service |
| 93870000 | Malignant neoplasm of liver (disorder) | Hepatic malignant neoplasm |
| 860691008 | Uses drug-eluting contraceptive implant (finding) | Uses subdermal contraceptive implant |
Reference sets
There are 55 reference sets in the October 2021 release.
For more information about New Zealand reference sets, please visit our website.
The New Zealand reference set list is available in Appendix 1, table 3.1.
Language reference sets
Language reference sets are created to define preferred terms and synonyms associated with each concept. There are three language reference sets in the October 2021 release.
Language Reference Set Name | SNOMED CT Concept ID (SCTID) | Members |
|---|---|---|
Maori [International Organization for Standardization 639-1 code mi] language reference set (foundation metadata concept) | 291000210106 | 157 |
New Zealand English language reference set (foundation metadata concept) | 271000210107 | 1155061 |
New Zealand English patient friendly terms language reference set (foundation metadata concept) | 281000210109 | 157 |
Maps
Five maps between SNOMED CT and other code systems are contained in the October 2021 release.
Map Name | SNOMED CT Concept ID (SCTID) | Members |
|---|---|---|
ACC translation table map (foundation metadata concept) | 401000210101 | 12795 |
MSD translation table map (foundation metadata concept) | 411000210104 | 58134 |
NZMT medicinal product to SNOMED CT map (foundation metadata concept) | 311000210107 | 2125 |
READ v2 to SNOMED CT map (foundation metadata concept) | 31000210106 | 88273 |
SNOMED CT to READ v2 map (foundation metadata concept) | 41000210103 | 66966 |
Technical notes
New Relationship Concrete Values files
Please be aware that the following new files have been added to the New Zealand Extension Release package, representing the new
Concrete Domains content being introduced in this editing cycle, in line with the related changes to the International Edition Release:
- sct2_RelationshipConcreteValues_Delta_NZ1000210_20211001.txt
- sct2_RelationshipConcreteValues_Snapshot_NZ1000210_20211001.txt
- sct2_RelationshipConcreteValues_Full_NZ1000210_20211001.txt
Known Issues
Known Issues are content or technical issues where the root cause is understood, and the resolution has been discussed and agreed but has yet to be implemented. This can be due to a number of reasons, from lack of capacity within the current editing cycle, to the risk of impact to the stability of SNOMED CT if the fix were to be deployed at that stage in the Product lifecycle.
The following known issues were identified, and agreed to be resolved in the next editing cycle:
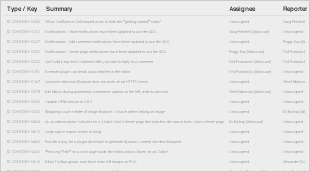
Appendix 1
Table 1.1. New concepts in SNOMED CT International Edition July 2021 release
SNOMED CT Concept ID (SCTID) | SNOMED CT Fully Specified Name (FSN) | Semantic tag |
|---|---|---|
1149100002 | Chronic Lymphocytic Leukemia International Prognostic Index (assessment scale) | Assessment scale |
1141914003 | Has firearms license (finding) | Finding |
1137691001 | Trying to give up using electronic cigarette (finding) | Finding |
1137692008 | Ex-electronic cigarette user for more than one year (finding) | Finding |
1137688001 | Ex-electronic cigarette user for less than one year (finding) | Finding |
1137690000 | Never used electronic cigarette (finding) | Finding |
1144648000 | Referral to pediatric ear, nose and throat service (procedure) | Procedure |
1157245009 | Fixed dose (qualifier value) | Qualifier value |
1149163003 | Revised International Staging System for multiple myeloma (staging scale) | Staging scale |
1149162008 | International Staging System for multiple myeloma (staging scale) | Staging scale |
1149164009 | Australian Clinicopathological Staging System for colorectal cancer (tumor staging) | Tumor staging |
1149131009 | Rai staging system for chronic lymphocytic leukemia (tumor staging) | Tumor staging |
1149099005 | Binet staging classification for chronic lymphocytic leukemia (tumor staging) | Tumor staging |
Table 2.1. New concepts in the SNOMED NZ Extension October 2021 release
| SNOMED CT Concept ID (SCTID) | SNOMED CT Fully Specified Name (FSN) | Semantic tag |
|---|---|---|
| 71701000210104 | Abnormal fetal blood sample (finding) | Finding |
| 71711000210102 | Assisted vaginal delivery for breech presentation (finding) | Finding |
| 72751000210109 | Contraception not required due to sterilization (finding) | Finding |
| 1137688001 | Ex-electronic cigarette user for less than one year (finding) | Finding |
| 1137692008 | Ex-electronic cigarette user for more than one year (finding) | Finding |
| 71861000210108 | Finding of assisted reproduction method of pregnancy (finding) | Finding |
| 71841000210107 | Hormonal stimulation fertilization pregnancy (finding) | Finding |
| 71851000210105 | Intrauterine insemination fertilization pregnancy (finding) | Finding |
| 72671000210109 | New Zealand alcohol consumption reference set (foundation metadata concept) | Foundation metadata concept |
| 72491000210106 | New Zealand current maternity reference set (foundation metadata concept) | Foundation metadata concept |
| 72621000210105 | New Zealand maternity Apgar score reference set (foundation metadata concept) | Foundation metadata concept |
| 72631000210107 | New Zealand maternity complementary therapies reference set (foundation metadata concept) | Foundation metadata concept |
| 72601000210102 | New Zealand maternity complications reference set (foundation metadata concept) | Foundation metadata concept |
| 72611000210100 | New Zealand maternity disorders reference set (foundation metadata concept) | Foundation metadata concept |
| 72661000210103 | New Zealand maternity family history reference set (foundation metadata concept) | Foundation metadata concept |
| 72591000210107 | New Zealand maternity findings reference set (foundation metadata concept) | Foundation metadata concept |
| 72481000210109 | New Zealand maternity history reference set (foundation metadata concept) | Foundation metadata concept |
| 72581000210105 | New Zealand maternity mode of delivery reference set (foundation metadata concept) | Foundation metadata concept |
| 72571000210108 | New Zealand maternity outcomes reference set (foundation metadata concept) | Foundation metadata concept |
| 72541000210103 | New Zealand maternity previous complications reference set (foundation metadata concept) | Foundation metadata concept |
| 72551000210100 | New Zealand maternity previous disorders reference set (foundation metadata concept) | Foundation metadata concept |
| 72531000210106 | New Zealand maternity previous findings reference set (foundation metadata concept) | Foundation metadata concept |
| 72521000210109 | New Zealand maternity previous mode of delivery reference set (foundation metadata concept) | Foundation metadata concept |
| 72511000210104 | New Zealand maternity previous outcomes reference set (foundation metadata concept) | Foundation metadata concept |
| 72501000210101 | New Zealand maternity previous procedures reference set (foundation metadata concept) | Foundation metadata concept |
| 72561000210102 | New Zealand maternity procedures reference set (foundation metadata concept) | Foundation metadata concept |
| 72471000210107 | New Zealand maternity reference set (foundation metadata concept) | Foundation metadata concept |
| 72641000210104 | New Zealand maternity screening and tests reference set (foundation metadata concept) | Foundation metadata concept |
| 72651000210101 | New Zealand maternity substances reference set (foundation metadata concept) | Foundation metadata concept |
| 72691000210108 | New Zealand non-medicinal drug reference set (foundation metadata concept) | Foundation metadata concept |
| 72681000210106 | New Zealand non-medicinal drug use reference set (foundation metadata concept) | Foundation metadata concept |
| 72731000210103 | New Zealand smoking intervention reference set (foundation metadata concept) | Foundation metadata concept |
| 72721000210100 | New Zealand vaping status reference set (foundation metadata concept) | Foundation metadata concept |
| 61451000210103 | Detection of Immunoglobulin A antibody to Severe acute respiratory syndrome coronavirus 2 nucleocapsid protein (observable entity) | Observable entity |
| 61441000210101 | Detection of Immunoglobulin A antibody to Severe acute respiratory syndrome coronavirus 2 spike protein (observable entity) | Observable entity |
| 61491000210105 | Detection of Immunoglobulin G antibody to Severe acute respiratory syndrome coronavirus 2 nucleocapsid protein (observable entity) | Observable entity |
| 61481000210108 | Detection of Immunoglobulin G antibody to Severe acute respiratory syndrome coronavirus 2 spike protein (observable entity) | Observable entity |
| 61471000210106 | Detection of Immunoglobulin M antibody to Severe acute respiratory syndrome coronavirus 2 nucleocapsid protein (observable entity) | Observable entity |
| 61461000210100 | Detection of Immunoglobulin M antibody to Severe acute respiratory syndrome coronavirus 2 spike protein (observable entity) | Observable entity |
| 61431000210109 | Detection of total antibodies to Severe acute respiratory syndrome coronavirus 2 nucleocapsid protein (observable entity) | Observable entity |
| 61421000210107 | Detection of total antibodies to Severe acute respiratory syndrome coronavirus 2 spike protein (observable entity) | Observable entity |
| 72761000210107 | Phlebotomy technician (occupation) | Occupation |
| 61511000210103 | Great aunt of subject (person) | Person |
| 61521000210108 | Great uncle of subject (person) | Person |
| 61551000210104 | Maternal aunt of subject (person) | Person |
| 61601000210101 | Maternal great grandfather of subject (person) | Person |
| 61591000210106 | Maternal great grandmother of subject (person) | Person |
| 61531000210105 | Maternal uncle of subject (person) | Person |
| 61541000210102 | Paternal aunt of subject (person) | Person |
| 61571000210107 | Paternal great grandfather of subject (person) | Person |
| 61581000210109 | Paternal great grandmother of subject (person) | Person |
| 61561000210101 | Paternal uncle of subject (person) | Person |
| 71981000210106 | Anterior episiotomy (procedure) | Procedure |
| 71831000210104 | J shape episiotomy (procedure) | Procedure |
| 71991000210108 | Mediolateral episiotomy (procedure) | Procedure |
| 71821000210101 | Midline episiotomy (procedure) | Procedure |
| 71721000210107 | Mifepristone induction of labor (procedure) | Procedure |
| 71731000210109 | Misoprostol induction of labor (procedure) | Procedure |
| 72781000210104 | Removal of products of conception from cervix (procedure) | Procedure |
| 71551000210107 | Ambulance service (qualifier value) | Qualifier value |
| 61741000210105 | Bone health service (qualifier value) | Qualifier value |
| 61761000210106 | Burns service (qualifier value) | Qualifier value |
| 71441000210105 | Cancer psychosocial service (qualifier value) | Qualifier value |
| 71511000210108 | Cardiorespiratory service (qualifier value) | Qualifier value |
| 61721000210104 | Child development service (qualifier value) | Qualifier value |
| 71471000210100 | Community musculoskeletal service (qualifier value) | Qualifier value |
| 71521000210103 | Continence service (qualifier value) | Qualifier value |
| 71491000210101 | Convalescent care service (qualifier value) | Qualifier value |
| 444913002 | Diabetes mellitus service (qualifier value) | Qualifier value |
| 61791000210101 | Employment support service (qualifier value) | Qualifier value |
| 61751000210108 | Fertility service (qualifier value) | Qualifier value |
| 61771000210100 | Fracture service (qualifier value) | Qualifier value |
| 71461000210106 | Maternity service with lead maternity carer (qualifier value) | Qualifier value |
| 71451000210108 | Maternity service without lead maternity carer (qualifier value) | Qualifier value |
| 61801000210102 | Metabolic medicine service (qualifier value) | Qualifier value |
| 61821000210105 | Neonatal surgical service (qualifier value) | Qualifier value |
| 61811000210100 | Pediatric diabetes service (qualifier value) | Qualifier value |
| 71541000210109 | Pediatric psychology service (qualifier value) | Qualifier value |
| 61731000210102 | Psychosocial service (qualifier value) | Qualifier value |
| 61781000210103 | Recreational therapy service (qualifier value) | Qualifier value |
| 71481000210103 | Smoking cessation service (qualifier value) | Qualifier value |
| 61831000210107 | Spinal surgery service (qualifier value) | Qualifier value |
| 71531000210101 | Wound care service (qualifier value) | Qualifier value |
| 52581000210108 | Breast metastatic capecitabine 1000 chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52471000210105 | Colorectal metastasic raltitrexed chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52261000210103 | Colorectal metastatic - modified fluorouracil, folinic acid, irinotecan and oxaliplatin low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52271000210109 | Colorectal metastatic - modified fluorouracil, folinic acid, irinotecan and oxaliplatin, high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52251000210101 | Colorectal metastatic - modified fluorouracil, folinic acid, irinotecan, oxaliplatin and bevacizumab low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52071000210102 | Colorectal metastatic capecitabine 1000 chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52061000210108 | Colorectal metastatic capecitabine 1250 chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52051000210105 | Colorectal metastatic capecitabine and bevacizumab chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52041000210107 | Colorectal metastatic capecitabine and mitomycin chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52081000210100 | Colorectal metastatic capecitabine and oxaliplatin chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52031000210104 | Colorectal metastatic capecitabine, oxaliplatin and bevacizumab chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52001000210109 | Colorectal metastatic cetuximab and irinotecan every two weeks chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52021000210101 | Colorectal metastatic cetuximab weekly chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52011000210106 | Colorectal metastatic cetuximab weekly and irinotecan every two weeks chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51911000210103 | Colorectal metastatic fluorouracil, folinic acid, and oxaliplatin chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51981000210109 | Colorectal metastatic irinotecan and oxaliplatin chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51991000210106 | Colorectal metastatic irinotecan every three weeks chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52591000210105 | Colorectal metastatic mDe Gramont high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52611000210103 | Colorectal metastatic mDe Gramont low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51971000210107 | Colorectal metastatic modified capecitabine and irinotecan chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51961000210101 | Colorectal metastatic modified fluorouracil , folinic acid, and irinotecan high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51881000210103 | Colorectal metastatic modified fluorouracil and folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52101000210105 | Colorectal metastatic modified fluorouracil, folinic acid and oxaliplatin high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52111000210107 | Colorectal metastatic modified fluorouracil, folinic acid and oxaliplatin low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51951000210104 | Colorectal metastatic modified fluorouracil, folinic acid, and irinotecan low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51941000210102 | Colorectal metastatic modified fluorouracil, folinic acid, irinotecan and bevacizumab high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51931000210105 | Colorectal metastatic modified fluorouracil, folinic acid, irinotecan and bevacizumab low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51921000210108 | Colorectal metastatic modified fluorouracil, folinic acid, irinotecan and cetuximab every two weeks high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52291000210108 | Colorectal metastatic modified fluorouracil, folinic acid, irinotecan and cetuximab weekly high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52281000210106 | Colorectal metastatic modified fluorouracil, folinic acid, irinotecan and cetuximab weekly low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52091000210103 | Colorectal metastatic modified fluorouracil, folinic acid, irrinotecan and cetuximab every two weeks low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52121000210102 | Colorectal metastatic modified fluorouracil, folinic acid, oxaliplatin and bevacizumab high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52131000210100 | Colorectal metastatic modified fluorouracil, folinic acid, oxaliplatin and bevacizumab low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52141000210108 | Colorectal metastatic modified fluorouracil, folinic acid, oxaliplatin and cetuximab every two weeks high dose folinic acid (regime/therapy) | Regime/therapy |
| 52151000210106 | Colorectal metastatic modified fluorouracil, folinic acid, oxaliplatin and cetuximab every two weeks low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52301000210107 | Colorectal metastatic modified fluorouracil, folinic acid, oxaliplatin, irinotecan and bevacizumab high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52241000210104 | Colorectal metastatic mRoswell Park high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52481000210107 | Colorectal metastatic mRoswell Park low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52501000210104 | Colorectal metastatic raltitrexed and oxaliplatin chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52161000210109 | Colorectal metastatic regorafenib chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52621000210108 | Colorectal non metastatic mRoswell Park low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52181000210101 | Colorectal non-metastatic capecitabine and oxaliplatin chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52171000210103 | Colorectal non-metastatic capecitabine chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51891000210101 | Colorectal non-metastatic fluorouracil and folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 51901000210100 | Colorectal non-metastatic modified fluorouracil and folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52191000210104 | Colorectal non-metastatic modified fluorouracil, folinic acid and oxaliplatin high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52201000210102 | Colorectal non-metastatic modified fluorouracil, folinic acid and oxaliplatin low dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52491000210109 | Colorectal non-metastatic mRoswell Park high dose folinic acid chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52631000210105 | Colorectal rectal metastatic - capecitabine chemoradiation 5 day dosing chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52601000210100 | Colorectal rectal metastatic fluorouracil chemoradiation chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52211000210100 | Colorectal rectal non-metastatic capecitabine chemoradiation 5 day dosing chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52221000210105 | Colorectal rectal non-metastatic capecitabine chemoradiation continuous dosing chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52231000210107 | Colorectal rectal non-metastatic fluorouracil chemoradiation chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52331000210102 | Lung Non Small Cell Lung Cancer metastatic alectinib chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52401000210103 | Lung Non Small Cell Lung Cancer non-metastatic alectinib chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52571000210106 | Respiratory mesothelioma metastatic - vinorelbine chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52551000210103 | Respiratory non small cell lung cancer metastic carboplatin and paclitaxel weekly chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52641000210102 | Respiratory non small cell lung cancer metastic carboplatin, paclitaxel, atezolizumab and bevacizumab chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52651000210104 | Respiratory non small cell lung cancer metastic carboplatin, paclitaxel, pembrolizumab chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52671000210107 | Respiratory non small cell lung cancer metastic carboplatin, pemetrexed and pembrolizumab chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52661000210101 | Respiratory non small cell lung cancer metastic crizotinib chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52451000210102 | Urogenital prostate castration resistant metastatic abiraterone and dexamethasone chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52321000210104 | Urogenital prostate castration resistant metastatic abiraterone and prednisone chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52311000210109 | Urogenital prostate castration resistant metastatic abiraterone and prednisone fed state chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52511000210102 | Urogenital prostate castration resistant metastatic cabazitaxel every three weeks and prednisone chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52561000210100 | Urogenital prostate castration resistant metastatic carboplatin and docetaxel chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52381000210103 | Urogenital prostate castration resistant metastatic docetaxel every three weeks and prednisone (regime/therapy) | Regime/therapy |
| 52391000210101 | Urogenital prostate castration resistant metastatic docetaxel and every two weeks prednisone chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52531000210109 | Urogenital prostate castration resistant metastatic enzalutamide chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52441000210100 | Urogenital prostate castration resistant non-metastatic apalutamide chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52371000210100 | Urogenital prostate castration sensitive metastatic abiraterone and prednisone chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52361000210106 | Urogenital prostate castration sensitive metastatic docetaxel every three weeks chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52421000210106 | Urogenital prostate metastatic bicalutamide chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52351000210108 | Urogenital prostate metastatic cyproterone chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52411000210101 | Urogenital prostate metastatic flutamide chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52461000210104 | Urogenital prostate metastatic goserelin chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52341000210105 | Urogenital prostate metastatic pembrolizumab flat dose chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52521000210107 | Urogenital prostate metastatic zoledronic acid every four weeks chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52541000210101 | Urogenital prostate metastatic zoledronic acid every twelve weeks chemotherapy regimen (regime/therapy) | Regime/therapy |
| 52431000210108 | Urogenital prostate sensitive metastatic apalutamide chemotherapy regimen (regime/therapy) | Regime/therapy |
| 72771000210101 | Contraception declined (situation) | Situation |
| 61611000210104 | Family history with explicit context pertaining to great aunt (situation) | Situation |
| 61621000210109 | Family history with explicit context pertaining to great uncle (situation) | Situation |
| 61641000210103 | Family history with explicit context pertaining to maternal aunt (situation) | Situation |
| 61691000210107 | Family history with explicit context pertaining to maternal great grandfather (situation) | Situation |
| 61681000210105 | Family history with explicit context pertaining to maternal great grandmother (situation) | Situation |
| 61631000210106 | Family history with explicit context pertaining to maternal uncle (situation) | Situation |
| 61651000210100 | Family history with explicit context pertaining to paternal aunt (situation) | Situation |
| 61661000210102 | Family history with explicit context pertaining to paternal great grandfather (situation) | Situation |
| 61671000210108 | Family history with explicit context pertaining to paternal great grandmother (situation) | Situation |
| 61701000210107 | Family history with explicit context pertaining to paternal uncle (situation) | Situation |
| 72701000210108 | History of abnormal fetal blood sample (situation) | Situation |
| 72131000210102 | History of abnormal fetal heart rate (situation) | Situation |
| 72321000210101 | History of abnormal weight loss (situation) | Situation |
| 71891000210103 | History of antenatal depression (situation) | Situation |
| 72331000210104 | History of autoimmune disorder (situation) | Situation |
| 72381000210100 | History of bacterial vaginosis (situation) | Situation |
| 72391000210103 | History of bicornuate uterus (situation) | Situation |
| 72031000210101 | History of breech presentation (situation) | Situation |
| 72351000210105 | History of carcinoma invasive of uterine cervix (situation) | Situation |
| 72361000210108 | History of cervical intraepithelial neoplasia grade 1 (situation) | Situation |
| 72371000210102 | History of cervical intraepithelial neoplasia grade 2 (situation) | Situation |
| 71581000210102 | History of classical cesarean section (situation) | Situation |
| 71601000210105 | History of combined spinal and epidural local anesthetic block (situation) | Situation |
| 72451000210104 | History of congenital uterine anomaly (situation) | Situation |
| 61351000210109 | History of disorder of fetal structure in previous pregnancy (situation) | Situation |
| 72401000210100 | History of endometriosis (situation) | Situation |
| 71611000210107 | History of epidural anesthesia (situation) | Situation |
| 71901000210102 | History of excessive vomiting in pregnancy (situation) | Situation |
| 72281000210108 | History of extreme prematurity of infant (situation) | Situation |
| 72061000210105 | History of failed induction of labor (situation) | Situation |
| 72171000210100 | History of fetal complication (situation) | Situation |
| 72071000210104 | History of fetal distress (situation) | Situation |
| 72121000210104 | History of fetal growth abnormality (situation) | Situation |
| 72231000210109 | History of fetal or neonatal effect of maternal transmission of substance (situation) | Situation |
| 71871000210102 | History of fetus with chromosomal abnormality (situation) | Situation |
| 72161000210106 | History of fetus with congenital anomaly (situation) | Situation |
| 71621000210102 | History of general anesthesia (situation) | Situation |
| 72421000210108 | History of gonorrhea (situation) | Situation |
| 72291000210105 | History of hypothermia of newborn (situation) | Situation |
| 71921000210105 | History of infection during labor (situation) | Situation |
| 71931000210107 | History of intrapartum hemorrhage (situation) | Situation |
| 72241000210101 | History of large for gestational age newborn (situation) | Situation |
| 71631000210100 | History of local anesthesia (situation) | Situation |
| 71641000210108 | History of local anesthetic intrathecal block (situation) | Situation |
| 71591000210100 | History of lower uterine segment caesarean section (situation) | Situation |
| 72001000210106 | History of malpresentation of fetus (situation) | Situation |
| 72191000210101 | History of meconium stained liquor (situation) | Situation |
| 72711000210105 | History of mental disorder (situation) | Situation |
| 71561000210105 | History of miscarriage in second trimester (situation) | Situation |
| 72301000210109 | History of moderate to late prematurity of infant (situation) | Situation |
| 72341000210107 | History of negative cervical Papanicolaou smear (situation) | Situation |
| 72271000210106 | History of neonatal cardiovascular disorder (situation) | Situation |
| 72211000210102 | History of neonatal encephalopathy (situation) | Situation |
| 72221000210107 | History of neonatal hypoglycemia (situation) | Situation |
| 71941000210104 | History of obstructed labor (situation) | Situation |
| 72081000210102 | History of obstructed labor due to fetal malposition (situation) | Situation |
| 72141000210105 | History of oligohydramnios (situation) | Situation |
| 71661000210109 | History of operation on breast (situation) | Situation |
| 71671000210103 | History of operation on female genital organs (situation) | Situation |
| 72111000210109 | History of perineal hematoma (situation) | Situation |
| 72151000210108 | History of polihydramnios (situation) | Situation |
| 72431000210105 | History of polycystic ovary syndrome (situation) | Situation |
| 71951000210101 | History of prolonged first stage of labor (situation) | Situation |
| 71911000210100 | History of prolonged pregnancy (situation) | Situation |
| 71971000210109 | History of prolonged rupture of membranes (situation) | Situation |
| 71961000210103 | History of prolonged second stage of labor (situation) | Situation |
| 71651000210106 | History of pudendal nerve block - transvaginal (situation) | Situation |
| 72251000210103 | History of respiratory distress syndrome in the newborn (situation) | Situation |
| 72091000210100 | History of seizure disorder (situation) | Situation |
| 71571000210104 | History of surgical termination of pregnancy (situation) | Situation |
| 72261000210100 | History of transitory tachypnea of newborn (situation) | Situation |
| 72041000210109 | History of transverse lie presentation (situation) | Situation |
| 72441000210102 | History of trichomonal vaginitis (situation) | Situation |
| 71881000210100 | History of unplanned pregnancy (situation) | Situation |
| 72051000210107 | History of unstable lie presentation (situation) | Situation |
| 72411000210103 | History of uterine leiomyoma (situation) | Situation |
| 72461000210101 | History of vaginospasm (situation) | Situation |
| 72311000210106 | History of very preterm maturity of infant (situation) | Situation |
| 71741000210101 | Neonatal resuscitation - successful (situation) | Situation |
| 71751000210103 | Neonatal resuscitation - unsuccessful (situation) | Situation |
| 71761000210100 | Neonatal resuscitation not performed (situation) | Situation |
| 72201000210104 | No history of neonatal complication (situation) | Situation |
| 72181000210103 | No history of postpartum complication (situation) | Situation |
| 72011000210108 | Pertussis vaccination given (situation) | Situation |
| 71771000210106 | Ultrasound scan declined (situation) | Situation |
| 61501000210100 | Oropharyngeal and anterior nares swab (specimen) | Specimen |
Table 3.1 Reference sets in the SNOMED NZ Extension October 2021 release
SNOMED CT Concept ID (SCTID) | SNOMED CT Fully Specified Name (FSN) | Members |
|---|---|---|
| 351000210106 | New Zealand adverse reaction manifestation refset (foundation metadata concept) | 1886 |
| 72671000210109 | New Zealand alcohol consumption reference set (foundation metadata concept) | 3 |
| 421000210109 | New Zealand ambulance clinical impression reference set (foundation metadata concept) | 176 |
| 61241000210100 | New Zealand biological substance common allergen reference set (foundation metadata concept) | 208 |
| 91000210107 | New Zealand cardiology reference set (foundation metadata concept) | 63 |
| 61251000210102 | New Zealand chemical substance common allergen reference set (foundation metadata concept) | 623 |
| 191000210102 | New Zealand child developmental services reference set (foundation metadata concept) | 137 |
| 241000210102 | New Zealand child health assessment reference set (foundation metadata concept) | 186 |
| 471000210108 | New Zealand clinical specialty reference set (foundation metadata concept) | 158 |
| 61271000210105 | New Zealand common pharmaceutical dose form reference set (foundation metadata concept) | 12 |
| 61281000210107 | New Zealand common pharmaceutical dose frequency reference set (foundation metadata concept) | 12 |
| 61291000210109 | New Zealand common pharmaceutical dose unit reference set (foundation metadata concept) | 11 |
| 61231000210108 | New Zealand COVID-19 adverse reaction event from immunisation reference set (foundation metadata concept) | 58 |
| 261000210101 | New Zealand disability reference set (foundation metadata concept) | 96 |
| 61261000210104 | New Zealand edible substance common allergen reference set (foundation metadata concept) | 420 |
| 61000210102 | New Zealand emergency care diagnosis reference set (foundation metadata concept) | 1448 |
| 71000210108 | New Zealand emergency care presenting complaint reference set (foundation metadata concept) | 155 |
| 321000210102 | New Zealand emergency care procedure reference set (foundation metadata concept) | 73 |
| 141000210106 | New Zealand endocrinology reference set (foundation metadata concept) | 263 |
| 181000210104 | New Zealand general paediatric reference set (foundation metadata concept) | 441 |
| 131000210103 | New Zealand general surgery reference set (foundation metadata concept) | 97 |
| 101000210108 | New Zealand gynaecology reference set (foundation metadata concept) | 143 |
| 451000210100 | New Zealand health occupation reference set (foundation metadata concept) | 84 |
| 461000210102 | New Zealand health service type reference set (foundation metadata concept) | 112 |
| 501000210102 | New Zealand laboratory order reference set (foundation metadata concept) | 956 |
| 72621000210105 | New Zealand maternity Apgar score reference set (foundation metadata concept) | 33 |
| 72631000210107 | New Zealand maternity complementary therapies reference set (foundation metadata concept) | 17 |
| 72601000210102 | New Zealand maternity complications reference set (foundation metadata concept) | 32 |
| 72611000210100 | New Zealand maternity disorders reference set (foundation metadata concept) | 46 |
| 72661000210103 | New Zealand maternity family history reference set (foundation metadata concept) | 12 |
| 72591000210107 | New Zealand maternity findings reference set (foundation metadata concept) | 125 |
| 72581000210105 | New Zealand maternity mode of delivery reference set (foundation metadata concept) | 4 |
| 72571000210108 | New Zealand maternity outcomes reference set (foundation metadata concept) | 7 |
| 72541000210103 | New Zealand maternity previous complications reference set (foundation metadata concept) | 45 |
| 72551000210100 | New Zealand maternity previous disorders reference set (foundation metadata concept) | 63 |
| 72531000210106 | New Zealand maternity previous findings reference set (foundation metadata concept) | 14 |
| 72521000210109 | New Zealand maternity previous mode of delivery reference set (foundation metadata concept) | 4 |
| 72511000210104 | New Zealand maternity previous outcomes reference set (foundation metadata concept) | 8 |
| 72501000210101 | New Zealand maternity previous procedures reference set (foundation metadata concept) | 22 |
| 72561000210102 | New Zealand maternity procedures reference set (foundation metadata concept) | 40 |
| 72641000210104 | New Zealand maternity screening and tests reference set (foundation metadata concept) | 21 |
| 72651000210101 | New Zealand maternity substances reference set (foundation metadata concept) | 13 |
| 391000210104 | New Zealand microorganism reference set (foundation metadata concept) | 18722 |
| 50931000210104 | New Zealand nephrology reference set (foundation metadata) | 99 |
| 72691000210108 | New Zealand non-medicinal drug reference set (foundation metadata concept) | 16 |
| 72681000210106 | New Zealand non-medicinal drug use reference set (foundation metadata concept) | 6 |
| 251000210104 | New Zealand notifiable disease reference set (foundation metadata concept) | 96 |
| 50941000210107 | New Zealand ophthalmology reference set (foundation metadata concept) | 64 |
| 121000210100 | New Zealand rheumatology reference set (foundation metadata concept) | 126 |
| 481000210105 | New Zealand site of care reference set (foundation metadata concept) | 8 |
| 72731000210103 | New Zealand smoking intervention reference set (foundation metadata concept) | 5 |
| 51000210100 | New Zealand smoking reference set (foundation metadata concept) | 42 |
| 72741000210106 | New Zealand smoking status reference set (foundation metadata concept) | 4 |
| 441000210103 | New Zealand surgical complication reference set (foundation metadata concept) | 147 |
| 72721000210100 | New Zealand vaping status reference set (foundation metadata concept) | 7 |
Approvals
Download .pdf here: |
Draft Amendment History
|
