Role
Clinical Reference Group
The role of the Clinical Reference Group is to provide a mechanism for sharing clinical expertise and experience, as it pertains to the enhancement of SNOMED CT’s clinical content. The group aims to create a virtual community focused on a clinical specialty topic area.
The group will provide a mechanism for:
- Sharing good practice
- Supporting discussion items
- Issue resolution
- Identification of international terminology requirements (new or revised)
- Identification of clinical focused derivatives (new or revised)
- Provision of expert advice
Clinical Coordination Group
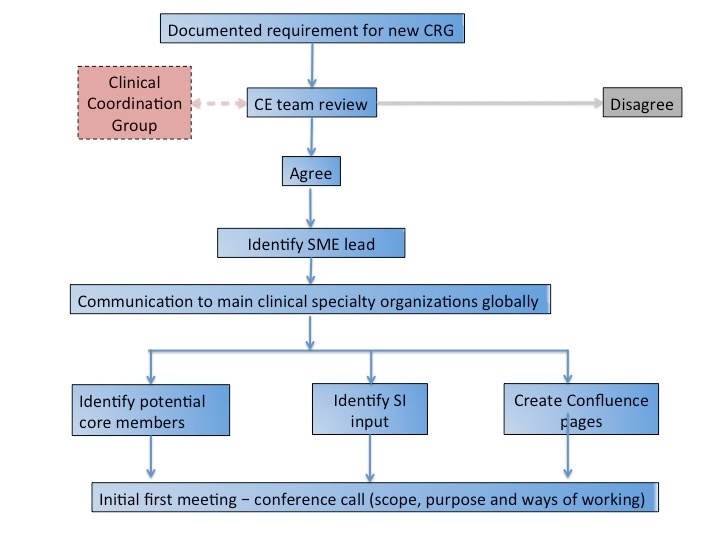
- Agree formation of a new Clinical Reference Group
- Review work requests from Clinical Reference Groups and provide recommendations
- Provide oversight and cross fertilization of discussions/activities across all Clinical Reference Groups
- Provide mechanism for reporting to the organisation on the issues raised through the Clinical Reference Groups
For more information on the Clinical Coordination Group click here
Accountability
Each Clinical Reference Group is accountable to the Clinical Coordination Group, comprised of members of the Clinical Engagement team and a Management Board member.
Membership
All Clinical Reference Groups have an open membership. Whilst a group may be targeted at a specific clinical specialty, there will also be the need to cover cross-cutting topics which will drawn in input from multiple specialties. Membership is open to anyone with an interest in that clinical area/topic. We actively support the inclusion of vendors and implementers as part of a group, to encourage the development of both content and derivative products that can easily and quickly be used within systems and meet requirements being identified.
Meetings
Whilst the Clinical Reference Groups by their very nature are virtual, SNOMED International will also support teleconferences on specific issues. Details of the process for doing this are included on each Clinical Reference Group Confluence page.
In addition, if the group identifies a requirement to meet face to face in order to deliver items specifically required for the SNOMED International workplan, this should be documented by the group and forwarded to the Clinical Coordination Group for consideration.
Group structure
The Clinical Reference Groups will operate predominately as a community of practice, supporting discussions on specific topics. As such, the group will have no formal chair. In the initial stages of the group, it is expected that discussion items will be generated by group members and by SNOMED International staff. Overtime the expectation is that individuals from within the group will take on a more active role identifying discussions items, although SNOMED International may still ask for expert advice and feedback from the group. Over time a leadership role may be identified as a requirement.
Clinical Reference Groups will be put in place where there is a clear use case requirement. Requirements will be reviewed by the Clinical Coordination Group, and the decision made on group creation. If over time the group becomes inactive, a decision will be made by the Clinical coordination Group whether to continue with support of the group, or whether to close the group.
Clinical Coordination Group
The Clinical Coordination Group will provide oversight and input into the Clinical Reference Groups. There are no formal reporting requirements between the Clinical Reference Group, however the Clinical Coordination Group will oversee and report on the activities of all Virtual Clinical groups to the SNOMED International Management Team. The Clinical Coordination Group will supply the Management Team with a quarterly review of the activities of all the Clinical Reference Groups, which will include recommendations for future work items identified by the Clinical Reference Groups. In addition, suggestions for work items from the Clinical Reference Groups will be reviewed by the Clinical Coordination Group, who will make recommendations on how to proceed with the suggestions. If the suggestion is to move forward as a formal work item, the Clinical Coordination Group will manage and support the process.
Openness and Transparency
The ways of working of the Clinical Reference Groups support inclusiveness, through the open membership and by encouraging all members to take a active role within group discussions.
All the Clinical Reference Groups are allocated a home page within Confluence. These pages are available to view by anyone, irrespective of whether you are logged into Confluence. However, to take an active part in the group discussions, individuals must have a Confluence account, and must be logged into Confluence. This ensure that all comments and discussions are attributed to the individual that created them.
International Perspective
The aim of all discussions in the Clinical Reference Groups are to provide an International perspective on a topic. Whilst discussions will obviously include examples and issues raised by members that arise from local/national perspectives, it should be understood that any requirements elicited from the group must provide an internationally accepted solution.
Moderation
The SNOMED International Clinical Engagement Team will work to provide any required moderation for Clinical Reference Groups, reviewing ongoing discussions, ensuring discussions are inclusive and dealing with SNOMED International specific points. If points are raised within discussions that are sensitive to individuals or organisations, SNOMED International reserve the right to discuss with the contributor and removing the point through mutual agreement.
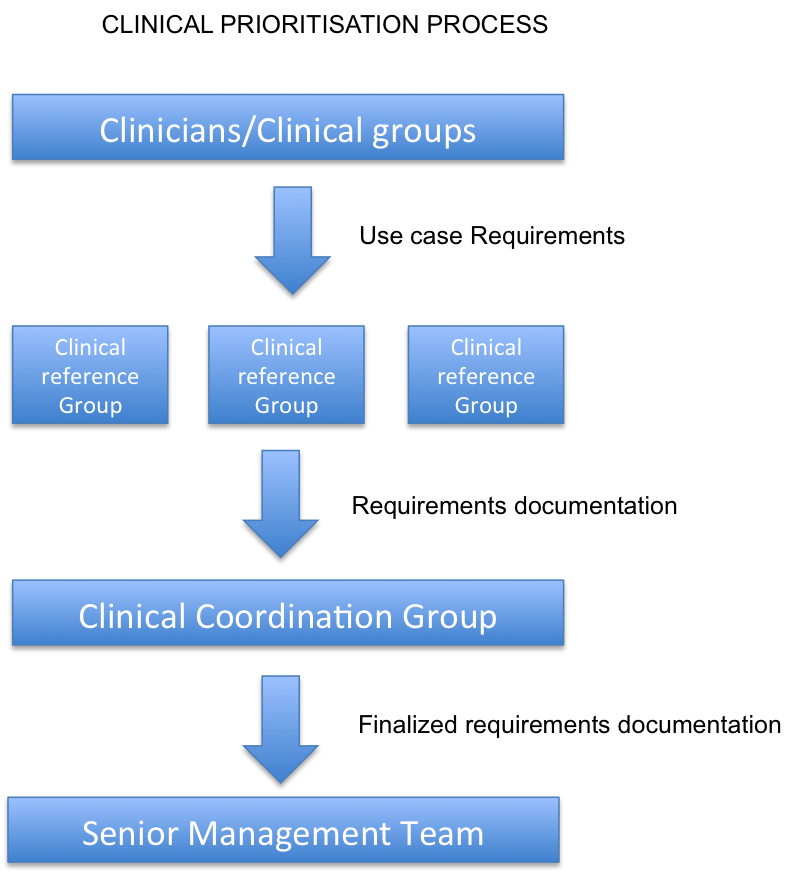
Prioritisation of clinical requirements
The following process will be followed for clinical priorities arising from the clinical community.
Note
These ways of working are expected to be revised on an ongoing basis as Clinical Reference Groups become more established, and we gain a greater understanding of internal and external drivers and gain feedback.