Definition of CD (precisely)
An abstract representation of a medicinal product based on description of 1) its precise active ingredient substances only and explicitly, 2) the stated basis of strength substance(s) with strength, expressed as presentation strength with unit of presentation or as concentration strength as appropriate, and 3) with its manufactured dose form (but in reconstituted oral liquid preparations, the administrable dose form - see below).
All Clinical Drugs that contain multiple active ingredient substances will have parent MPF and MP concepts that have the same set of active ingredient substances.
The limitation of the Clinical Drug class to the closed world view by the description of its precise active ingredient substances only precludes description of excipient substances such as flavours, preservatives, sweeteners etc as ingredients in a Clinical Drug. These substances can have significance for allergies etc. but can only be reliably described for individual authorised manufactured products, and as such are not within the scope of the international edition.
Similarly, by limiting the the Clinical Drug class in the international edition to expression of strength either as concentration strength or as presentation strength, medicinal product concepts that could usefully have both concentration and presentation strength (for example some liquid products such as liquid parenteral products or liquids for inhalation via a nebuliser) will have only concentration strength in the international edition. National extensions may author clinical drug concepts using the presentation strength(s) and unit(s) of presentation available in their jurisdiction if use case(s) require this. These concepts will be child concepts of the concentration clinical drug in the international edition. The diagrams below illustrate this:

Figure 28: Clinical Drug concepts and their relationship together and to MPF only concepts

Figure 29: Clinical Drug concepts and their relationship together and to MPF only concepts in SNOMED notation, showing optional national extension concepts
Example:

Figure 30: Clinical Drug example showing optional national extension concepts
Please also reference the National Extension specification for how to use additional model attributes to fully define concepts that can have both a concentration strength and a presentation strength such that they classify correctly.
Use cases supported by CD (precisely)
Use cases supported by the clinical drug concept include:
- As the abstract representation of products that are authorised, although without any sense of the excipient substances, the clinical drug concept is the source from which all other representation of medicinal product concepts flows; it acts as a clinically relevant grouper concept for medicinal products, and as such can support
- international cross-border care delivery
- International and national interoperability of patient medication information such as in patient summaries
- In national extensions, for many clinical purposes, such as product prescribing, adverse event reporting, formulary management, in recording medication history and in medication profiles
- Internationally and nationally in decision support and in protocols and treatment guidelines, when a more complete description of a product is required than MP or MPF
- In pharmacovigilance, especially for description of concomitant medication
- In analysis and research
Availability of CD concepts in the international edition
This class forms part of the medicinal product content provided in the international edition, although for liquid products, only concentration strength representation is provided.
Clinical Drug (precisely) (presentation strength)
Definition of Clinical Drug (precisely) (presentation strength)
An abstract representation of a medicinal product based on description of 1) its precise active ingredient substances only and explicitly, 2) the stated basis of strength substance(s) with strength, expressed as presentation strength with unit of presentation and 3) with its manufactured dose form.
This is used for product types such as tablets, capsules, pessaries, suppositories (Strength Pattern 1a in Appendix A: Product Patterns), sachets, ampoules or vials containing powders or granules etc. (solid dosage forms) and those presented with a metered dose valve such as inhalers and sprays.
Example diagrams for CD (precisely) presentation strength
Stated template view:
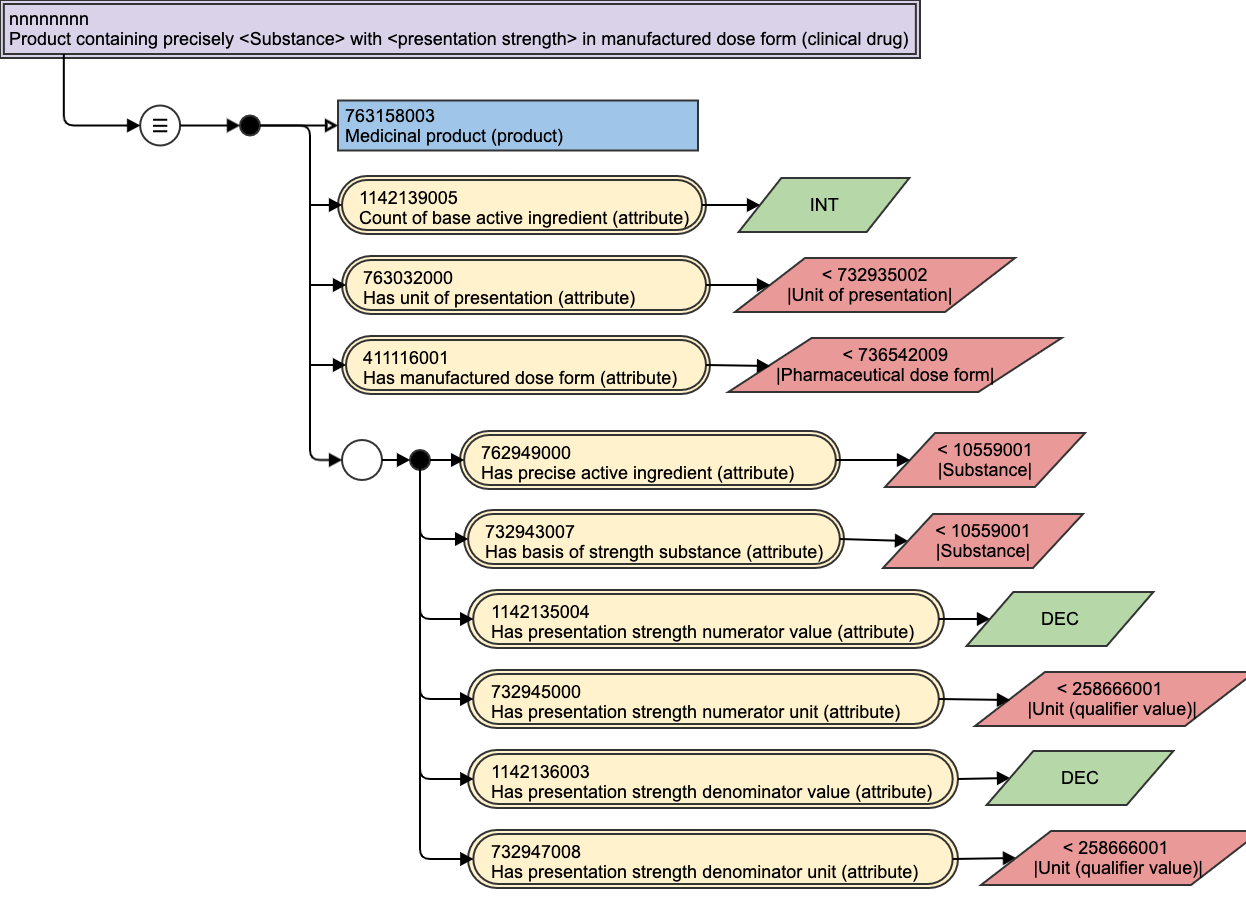
Figure 31: Clinical drug, presentation strength, stated template view
Examples: single active ingredient product: stated view followed by the inferred view that shows the proximal parent concepts associated with the product:
Figure 32: Clinical drug, presentation strength, example stated view
Figure 33: Clinical drug, presentation strength, example inferred view
Attributes of CD (precisely) (presentation strength)
The Clinical Drug (CD precisely) (presentation) concept is defined by three attributes and a set of substance/strength attributes:
| Semantic tag | (clinical drug) | |
| Definition status | ||
Attribute 411116001 |Has manufactured dose form| | Range Cardinality
Notes
| |
Attribute 763032000 |Has unit of presentation| | Range Cardinality
Notes
| |
Range
Cardinality
Note
| ||
Role Group One role group is required for each precise active ingredient | Range Cardinality (within role group)
Notes
| |
Range Cardinality (within role group)
Notes
| ||
Range
Cardinality (within role group)
| ||
Range
Cardinality (within role group)
| ||
For concepts that have two or more active ingredient substances that are modifications of the same base substance and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required in order to support correct relationships generated by the MRCM:
Range
Cardinality
|
For concepts that have two or more active ingredient substances that are modifications of the same base active ingredient substance (i.e. parent ingredient substance) and where one is a further modification of the other (for example, a multi-ingredient product containing both dexamethasone phosphate and dexamethasone sodium phosphate, where the dexamethasone phosphate is a modification of dexamethasone (base) and dexamethasone sodium phosphate is a further modification of the dexamethasone phosphate) and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required in order to support correct relationships generated by the MRCM:
Attribute 1142140007 |Count of active ingredient| | Range
Cardinality
|
As described in the MRCM rules, for practical and pragmatic reasons the additional ingredient count attributes have to be applied iteratively based on requirement.
Clinical Drug (precisely) (concentration strength)
Definition of Clinical Drug (precisely) (concentration strength)
An abstract representation of a medicinal product based on description of 1) its precise active ingredient substances only and explicitly, 2) the stated basis of strength substance(s) with strength, expressed as concentration strength and 3) with its manufactured dose form (with the exception of reconstituted oral liquid preparations, where the administrable dose form is be used as it is the most clinically relevant),
This is used for product types such as cutaneous semi-solids (without metered actuation), bulk powders and granules, topical liquids (without metered actuation) including drops, oral liquids and drops, nebuliser liquids and liquid parenteral products.
Example diagrams for CD (precisely) concentration strength
Stated template view:

Figure 34: Clinical drug, concentration strength, stated template view
Examples: single active ingredient product: stated view followed by the inferred view that shows the proximal parent concepts associated with the product:
Figure 35: Clinical drug, concentration strength, example stated view
Figure 36: Clinical drug, concentration strength, example inferred view
Attributes of CD (precisely) (concentration strength)
The Clinical Drug (CD precisely) (concentration) concept is defined by two attributes and a set of substance/strength attributes; a Clinical Drug described only by concentration strength does not have a unit of presentation:
| Semantic tag | (clinical drug) | |
| Definition status | ||
Attribute 411116001 |Has manufactured dose form| | Range Cardinality
Notes
| |
Range
Cardinality
Note
| ||
Role Group One role group is required for each precise active ingredient | Range Cardinality (within role group)
Notes
| |
Range Cardinality (within role group)
Notes
| ||
Range
Cardinality (within role group)
| ||
Range
Cardinality (within role group)
| ||
For concepts that have two or more active ingredient substances that are modifications of the same base substance and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required n order to support correct relationships generated by the MRCM:
Range
Cardinality
|
For concepts that have two or more active ingredient substances that are modifications of the same base active ingredient substance (i.e. parent ingredient substance) and where one is a further modification of the other (for example, a multi-ingredient product containing both dexamethasone phosphate and dexamethasone sodium phosphate, where the dexamethasone phosphate is a modification of dexamethasone (base) and dexamethasone sodium phosphate is a further modification of the dexamethasone phosphate) and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required n order to support correct relationships generated by the MRCM:
Attribute 1142140007 |Count of active ingredient| | Range
Cardinality
|
As described in the MRCM rules, for practical and pragmatic reasons the additional ingredient count attributes have to be applied iteratively based on requirement.
Other Clinical Drug Grouping Concepts not present in this model or in the international edition
Clinical Drug concepts in the international release are defined by their precise active ingredient substance(s) and their basis of strength substance, as described above. A concept that grouped clinical drugs by their strength and basis of strength substance only (i.e. disregarding the precise active ingredient substance) may be appropriate in some contexts in national extensions (e.g. 'amlodipine 10mg conventional release oral tablet' as a concept with three child concepts 'amlodipine (as amlodipine besiliate) 10mg conventional release oral tablet' and 'amlodipine (as amlodipine mesiliate) 10mg conventional release oral tablet' 'amlodipine (as amlodipine maleate) 10mg conventional release oral tablet'. A Clinical Drug grouping concept of this nature would be a "Basis of Strength Substance Clinical Drug" concept as opposed to a "Clinical Drug containing Precisely" concept.
IDMP Compatibility
Although a Clinical Drug might look directly compatible with the IDMP concept of "Manufactured Item", in IDMP, a Manufactured Item is an "actual manufactured item (the tablet, liquid, cream contained within the package) as it is delivered from the manufacturer but before any transformation, if applicable, for administration to or use by the patient"; it is therefore a representation of a real entity, rather than an abstract entity. They are therefore not directly compatible classes of entities. A Manufactured Item is described by substances in a variety of roles, including excipient substances, not just its active substance(s) and their strengths. A Manufactured Item can be related to an appropriate Clinical Drug on the basis of its active ingredient substance(s) and relevant strength so that the Clinical Drug being an abstracted representation of the Manufactured Item, but they are not equivalent. The Manufactured Item concept in IDMP is equivalent to the Real Clinical Drug concept of the SNOMED national extension model.
On the basis that the IDMP concept of a Pharmaceutical Product could be defined by substance(s) playing only an active ingredient role, then the Clinical Drug concept is more directly compatible with the IDMP Pharmaceutical Product concept however if it is intended that the IDMP Pharmaceutical Product does include excipient substances, then there is no compatibility. Even then, the IDMP Pharmaceutical Product concept is clear that the dose form attribute is populated by the administrable dose form rather than the manufactured dose form, whereas, for everything other than products presented as reconstituted oral liquids, the Clinical Drug uses the manufactured dose form. Although for probably the majority of medicinal products the manufactured dose form is also the administrable dose form, for the minority for which this is not the case (for example, parenteral products presented as powders or granules that must be dissolved or dispersed prior to administration to the patient) this difference is significant. It is therefore not possible to state any direct class level equivalence between a Clinical Drug and an IDMP Pharmaceutical Product.
There is some compatibility between an IDMP PhP4 concept and a Clinical Drug. However, It is not (yet) clear as to how the "active substance - strength" description will be described in IDMP implementation. The SNOMED Clinical Drug is explicit in stating the basis of strength substance in its relevant granularity as required for patient care; IDMP is currently less clear as to how that will be done and what effect that will have on the description of a Pharmaceutical Product. In addition, IDMP allows for "active substance - strength" to be described by using either (and possibly even both) a Substance and Specified Substance. The distinction between Substance and Specified Substance in IDMP is thus: a substance is "any matter of defined composition that has discrete existence, whose origin may be biological, mineral or chemical" whereas a Specified Substance is one that is "defined by groups of elements that describes multi-substance materials or specifies further information on substances relevant to the description of Medicinal Products". Specified substances are substances like simeticone, which are mixture substances, or substances that are defined by pharmacopoeial specification (like water for injection) or substance where a particular manufacturing process is specified (as for biosimilar products). For SNOMED CT, all such substances, with the possible exception of 'water for injection') could be present in the Substance hierarchy and are therefore candidate concepts to be used in the ingredient role attributes of concepts in the Medicinal Product hierarchy; as such the IDMP distinction between Substance and Specified Substance has no material effect.
Definition of CD (precisely)
An abstract representation of a medicinal product based on description of 1) its precise active ingredient substances only and explicitly, 2) the stated basis of strength substance(s) with strength, expressed as presentation strength with unit of presentation or as concentration strength as appropriate, and 3) with its manufactured dose form (but in reconstituted oral liquid preparations, the administrable dose form - see below).
All Clinical Drugs that contain multiple active ingredient substances will have parent MPF and MP concepts that have the same set of active ingredient substances.
The limitation of the Clinical Drug class to the closed world view by the description of its precise active ingredient substances only precludes description of excipient substances such as flavours, preservatives, sweeteners etc as ingredients in a Clinical Drug. These substances can have significance for allergies etc. but can only be reliably described for individual authorised manufactured products, and as such are not within the scope of the international edition.
Similarly, by limiting the the Clinical Drug class in the international edition to expression of strength either as concentration strength or as presentation strength, medicinal product concepts that could usefully have both concentration and presentation strength (for example some liquid products such as liquid parenteral products or liquids for inhalation via a nebuliser) will have only concentration strength in the international edition. National extensions may author clinical drug concepts using the presentation strength(s) and unit(s) of presentation available in their jurisdiction if use case(s) require this. These concepts will be child concepts of the concentration clinical drug in the international edition. The diagrams below illustrate this:

Figure 37: Clinical Drug concepts and their relationship together and to MPF only concepts

Figure 38: Clinical Drug concepts and their relationship together and to MPF only concepts in SNOMED notation, showing optional national extension concepts
Example:

Figure 39: Clinical Drug example showing optional national extension concepts
Please also reference the National Extension specification for how to use additional model attributes to fully define concepts that can have both a concentration strength and a presentation strength such that they classify correctly.
Use cases supported by CD (precisely)
Use cases supported by the clinical drug concept include:
- As the abstract representation of products that are authorised, although without any sense of the excipient substances, the clinical drug concept is the source from which all other representation of medicinal product concepts flows; it acts as a clinically relevant grouper concept for medicinal products, and as such can support
- international cross-border care delivery
- International and national interoperability of patient medication information such as in patient summaries
- In national extensions, for many clinical purposes, such as product prescribing, adverse event reporting, formulary management, in recording medication history and in medication profiles
- Internationally and nationally in decision support and in protocols and treatment guidelines, when a more complete description of a product is required than MP or MPF
- In pharmacovigilance, especially for description of concomitant medication
- In analysis and research
Availability of CD concepts in the international edition
This class forms part of the medicinal product content provided in the international edition, although for liquid products, only concentration strength representation is provided.
Clinical Drug (precisely) (presentation strength)
Definition of Clinical Drug (precisely) (presentation strength)
An abstract representation of a medicinal product based on description of 1) its precise active ingredient substances only and explicitly, 2) the stated basis of strength substance(s) with strength, expressed as presentation strength with unit of presentation and 3) with its manufactured dose form.
This is used for product types such as tablets, capsules, pessaries, suppositories (Strength Pattern 1a in Appendix A: Product Patterns), sachets, ampoules or vials containing powders or granules etc. (solid dosage forms) and those presented with a metered dose valve such as inhalers and sprays.
Example diagrams for CD (precisely) presentation strength
Stated template view:

Figure 40: Clinical drug, presentation strength, stated template view
Examples: single active ingredient product: stated view followed by the inferred view that shows the proximal parent concepts associated with the product:
Figure 41: Clinical drug, presentation strength, example stated view
Figure 42: Clinical drug, presentation strength, example inferred view
Attributes of CD (precisely) (presentation strength)
The Clinical Drug (CD precisely) (presentation) concept is defined by three attributes and a set of substance/strength attributes:
| Semantic tag | (clinical drug) | |
| Definition status | ||
Attribute 411116001 |Has manufactured dose form| | Range Cardinality
Notes
| |
Attribute 763032000 |Has unit of presentation| | Range Cardinality
Notes
| |
Range
Cardinality
Note
| ||
Role Group One role group is required for each precise active ingredient | Range Cardinality (within role group)
Notes
| |
Range Cardinality (within role group)
Notes
| ||
Range
Cardinality (within role group)
| ||
Range
Cardinality (within role group)
| ||
For concepts that have two or more active ingredient substances that are modifications of the same base substance and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required in order to support correct relationships generated by the MRCM:
Range
Cardinality
|
For concepts that have two or more active ingredient substances that are modifications of the same base active ingredient substance (i.e. parent ingredient substance) and where one is a further modification of the other (for example, a multi-ingredient product containing both dexamethasone phosphate and dexamethasone sodium phosphate, where the dexamethasone phosphate is a modification of dexamethasone (base) and dexamethasone sodium phosphate is a further modification of the dexamethasone phosphate) and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required in order to support correct relationships generated by the MRCM:
Attribute 1142140007 |Count of active ingredient| | Range
Cardinality
|
As described in the MRCM rules, for practical and pragmatic reasons the additional ingredient count attributes have to be applied iteratively based on requirement.
Clinical Drug (precisely) (concentration strength)
Definition of Clinical Drug (precisely) (concentration strength)
An abstract representation of a medicinal product based on description of 1) its precise active ingredient substances only and explicitly, 2) the stated basis of strength substance(s) with strength, expressed as concentration strength and 3) with its manufactured dose form (with the exception of reconstituted oral liquid preparations, where the administrable dose form is be used as it is the most clinically relevant),
This is used for product types such as cutaneous semi-solids (without metered actuation), bulk powders and granules, topical liquids (without metered actuation) including drops, oral liquids and drops, nebuliser liquids and liquid parenteral products.
Example diagrams for CD (precisely) concentration strength
Stated template view:

Figure 43: Clinical drug, concentration strength, stated template view
Examples: single active ingredient product: stated view followed by the inferred view that shows the proximal parent concepts associated with the product:
Figure 44: Clinical drug, concentration strength, example stated view
Figure 45: Clinical drug, concentration strength, example inferred view
Attributes of CD (precisely) (concentration strength)
The Clinical Drug (CD precisely) (concentration) concept is defined by two attributes and a set of substance/strength attributes; a Clinical Drug described only by concentration strength does not have a unit of presentation:
| Semantic tag | (clinical drug) | |
| Definition status | ||
Attribute 411116001 |Has manufactured dose form| | Range Cardinality
Notes
| |
Range
Cardinality
Note
| ||
Role Group One role group is required for each precise active ingredient | Range Cardinality (within role group)
Notes
| |
Range Cardinality (within role group)
Notes
| ||
Range
Cardinality (within role group)
| ||
Range
Cardinality (within role group)
| ||
For concepts that have two or more active ingredient substances that are modifications of the same base substance and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required n order to support correct relationships generated by the MRCM:
Range
Cardinality
|
For concepts that have two or more active ingredient substances that are modifications of the same base active ingredient substance (i.e. parent ingredient substance) and where one is a further modification of the other (for example, a multi-ingredient product containing both dexamethasone phosphate and dexamethasone sodium phosphate, where the dexamethasone phosphate is a modification of dexamethasone (base) and dexamethasone sodium phosphate is a further modification of the dexamethasone phosphate) and where MP precisely concepts are required, and for single ingredient product concepts where the active substance is an ingredient in these multiple modification multi-ingredient products, the following extra ingredient count attribute will be required n order to support correct relationships generated by the MRCM:
Attribute 1142140007 |Count of active ingredient| | Range
Cardinality
|
As described in the MRCM rules, for practical and pragmatic reasons the additional ingredient count attributes have to be applied iteratively based on requirement.
Other Clinical Drug Grouping Concepts not present in this model or in the international edition
Clinical Drug concepts in the international release are defined by their precise active ingredient substance(s) and their basis of strength substance, as described above. A concept that grouped clinical drugs by their strength and basis of strength substance only (i.e. disregarding the precise active ingredient substance) may be appropriate in some contexts in national extensions (e.g. 'amlodipine 10mg conventional release oral tablet' as a concept with three child concepts 'amlodipine (as amlodipine besiliate) 10mg conventional release oral tablet' and 'amlodipine (as amlodipine mesiliate) 10mg conventional release oral tablet' 'amlodipine (as amlodipine maleate) 10mg conventional release oral tablet'. A Clinical Drug grouping concept of this nature would be a "Basis of Strength Substance Clinical Drug" concept as opposed to a "Clinical Drug containing Precisely" concept.
IDMP Compatibility
Although a Clinical Drug might look directly compatible with the IDMP concept of "Manufactured Item", in IDMP, a Manufactured Item is an "actual manufactured item (the tablet, liquid, cream contained within the package) as it is delivered from the manufacturer but before any transformation, if applicable, for administration to or use by the patient"; it is therefore a representation of a real entity, rather than an abstract entity. They are therefore not directly compatible classes of entities. A Manufactured Item is described by substances in a variety of roles, including excipient substances, not just its active substance(s) and their strengths. A Manufactured Item can be related to an appropriate Clinical Drug on the basis of its active ingredient substance(s) and relevant strength so that the Clinical Drug being an abstracted representation of the Manufactured Item, but they are not equivalent. The Manufactured Item concept in IDMP is equivalent to the Real Clinical Drug concept of the SNOMED national extension model.
On the basis that the IDMP concept of a Pharmaceutical Product could be defined by substance(s) playing only an active ingredient role, then the Clinical Drug concept is more directly compatible with the IDMP Pharmaceutical Product concept however if it is intended that the IDMP Pharmaceutical Product does include excipient substances, then there is no compatibility. Even then, the IDMP Pharmaceutical Product concept is clear that the dose form attribute is populated by the administrable dose form rather than the manufactured dose form, whereas, for everything other than products presented as reconstituted oral liquids, the Clinical Drug uses the manufactured dose form. Although for probably the majority of medicinal products the manufactured dose form is also the administrable dose form, for the minority for which this is not the case (for example, parenteral products presented as powders or granules that must be dissolved or dispersed prior to administration to the patient) this difference is significant. It is therefore not possible to state any direct class level equivalence between a Clinical Drug and an IDMP Pharmaceutical Product.
There is some compatibility between an IDMP PhP4 concept and a Clinical Drug. However, It is not (yet) clear as to how the "active substance - strength" description will be described in IDMP implementation. The SNOMED Clinical Drug is explicit in stating the basis of strength substance in its relevant granularity as required for patient care; IDMP is currently less clear as to how that will be done and what effect that will have on the description of a Pharmaceutical Product. In addition, IDMP allows for "active substance - strength" to be described by using either (and possibly even both) a Substance and Specified Substance. The distinction between Substance and Specified Substance in IDMP is thus: a substance is "any matter of defined composition that has discrete existence, whose origin may be biological, mineral or chemical" whereas a Specified Substance is one that is "defined by groups of elements that describes multi-substance materials or specifies further information on substances relevant to the description of Medicinal Products". Specified substances are substances like simeticone, which are mixture substances, or substances that are defined by pharmacopoeial specification (like water for injection) or substance where a particular manufacturing process is specified (as for biosimilar products). For SNOMED CT, all such substances, with the possible exception of 'water for injection') could be present in the Substance hierarchy and are therefore candidate concepts to be used in the ingredient role attributes of concepts in the Medicinal Product hierarchy; as such the IDMP distinction between Substance and Specified Substance has no material effect.
Feedback



