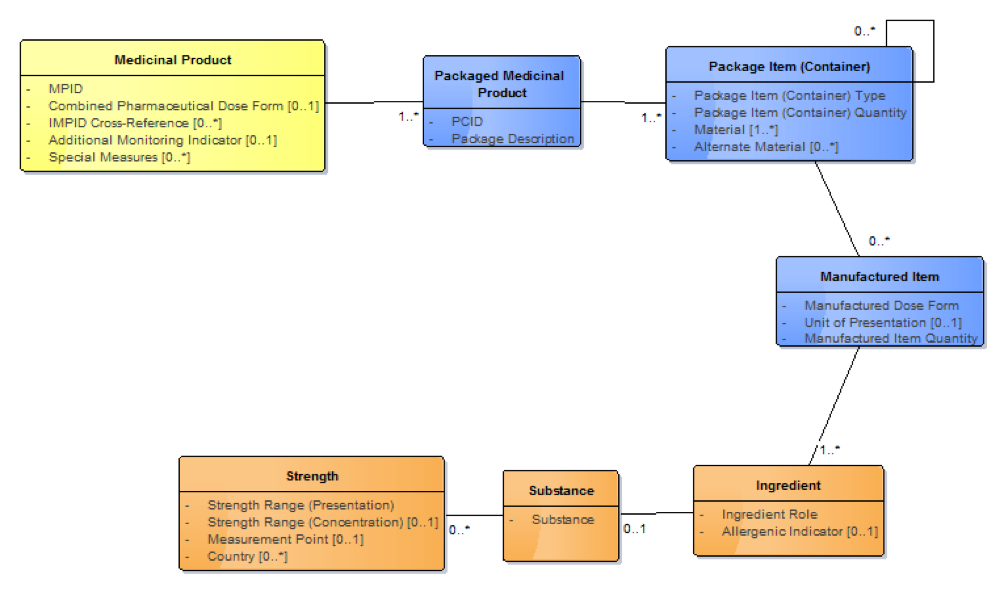
To correctly interpret "one countable instance of a whole of medicinal product", it is important that this is seen in the context of the overall description of a medicinal product, which is always presented from the manufacturer in as a "packaged medicinal product". Note that description of packaged medicinal products is outside the scope of the international release but may be included within a national extension. The IDMP Medicinal Product model describes this, showing how the Manufactured Item is related to the Packaged Medicinal Product via the Package Item (Container); this is a recursive class that represents both the package as supplied by the manufacturer (and, for example, labelled with the GTIN, the batch number and the expiry), and through a recursive relationship, with any sub-packages inside the outer pack.
By describing the various standard patterns of products with their basic dose forms and intimate containers, consistent representation of strength based on unit of presentation can be maintained.Note: in all the patterns described below, although pack size may be mentioned, this is to show how information is sourced from "what is". Description of pack size is out of scope for the international release, although it will be in scope for the national extension model, as some nations may require medicinal products that include description of pack size for their national terminology; therefore it is useful to have it shown here for informational purposes only.
Examples: various tablets, capsules, cachets, pessaries, suppositories, tampons
The unit of presentation is usually a less granular term than the manufactured dose form, and often corresponds to the basic dose form.
Strength is expressed as "per one unit of presentation" and the presentation strength and the concentration are exactly the same.
Example 1: A bottle of 56 simvastatin 40mg oral tablets
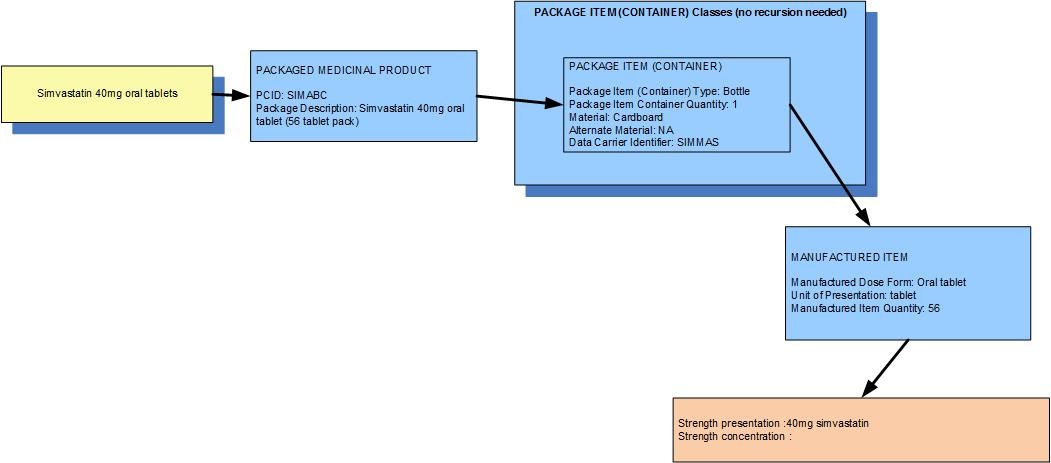
| Manufactured dose form | Oral tablet |
|
| Unit of presentation | Tablet |
|
| [Pack size] | 56 tablets in the container
|
|
| Precise active ingredient | simvastatin |
|
| Basis of strength substance | simvastatin |
|
| Presentation strength (logical) | 40 mg per 1 unit of presentation |
|
| Presentation strength | 40 mg [per 1 tablet] | UCUM: 40 mg [per 1 each] |
| Concentration strength |
| The weight of the tablet is not usually known so concentration strength is not usually available and is not deemed clinically significant |
Example 2: A pack of 28 bisoprolol 5 mg oral tablets contains two blister strips each with 14 tablets
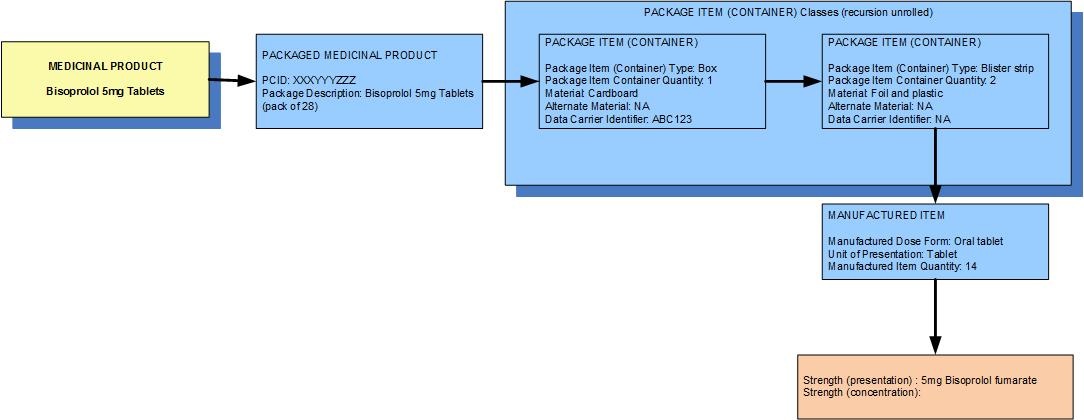
| Manufactured dose form | Oral tablet |
|
| Unit of presentation | Tablet |
|
| [Pack size] | 14 tablets in the blister strip2 blister strips in the box | 28 tablets in the outer container |
| Precise active ingredient | bisoprolol fumarate |
|
| Basis of strength substance | bisoprolol fumarate |
|
| Presentation strength (logical) | 5 mg per 1 unit of presentation |
|
| Presentation strength | 5 mg [per 1 tablet] | UCUM: 5 mg [per 1 each] |
| Concentration strength |
| The weight of the tablet is not usually known so concentration strength is not usually available and is not deemed clinically significant |
Continuous presentation: Metered dose unit of presentation
Examples: various inhalers, nasal sprays, some cutaneous sprays/foamsThe unit of presentation is the actuation, the “single operation of a metered-dose pump, valve or other equivalent dosing mechanism” [EDQM].Strength is expressed as “per one unit of presentation” and the presentation strength and the concentration are exactly the same.
Example: A single inhaler containing a total of 200 actuations worth of salbutamol, 100 micrograms per actuation, packaged in a box
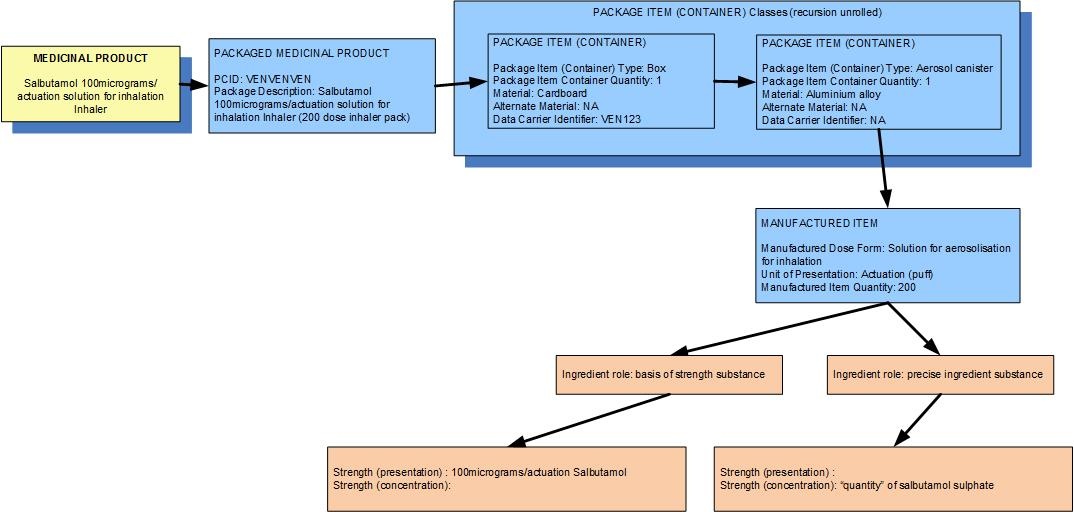
| Manufactured dose form | Solution for aerosolisation |
|
| Unit of presentation | Actuation |
|
| [Pack size] | 200 actuations in the inhaler |
|
| Precise active ingredient | salbutamol sulphate |
|
| Basis of strength substance | salbutamol |
|
| Presentation strength (logical) | 100 mcg per 1 unit of presentation |
|
| Presentation strength | 100 mcg per 1 actuation | UCUM: 100 mcg per 1 each |
| Concentration strength |
| The concentration of salbutamol sulphate in the inhalant solution inside the inhaler container is probably known to the regulatory agency but is not deemed clinically significant |
Continuous presentation: Oral liquids designed for administration by "metered" medicine spoon
Examples: oral solutions, suspensions, emulsions, syrups. This is a variation on the metered dose presentation; the unit of presentation supplied by the manufacturer to provide the “metered dose” is the 5mL spoonful, since this represents “the quantity of product that is administered by filling a single spoon administration device” [EDQM]. Strength is expressed as “per one unit of presentation” (per 5 mL [spoonful]) BUT the presentation strength and the concentration are NOT the same, since these are continuous liquids, so the concentration strength of “per 1 mL” will usually be a different value. Note that explicit representation of the medicine spoon would be as an administration device, and is therefore out of scope of the international Medicinal Product hierarchy. National extensions may wish to represent the inclusion of a medicine spoon (or indeed any other administration device such as an applicator) in the package description (as in IDMP, for example) should the use case(s) require.
Example: A bottle of 125 mL of aciclovir oral suspension 200mg/5mL
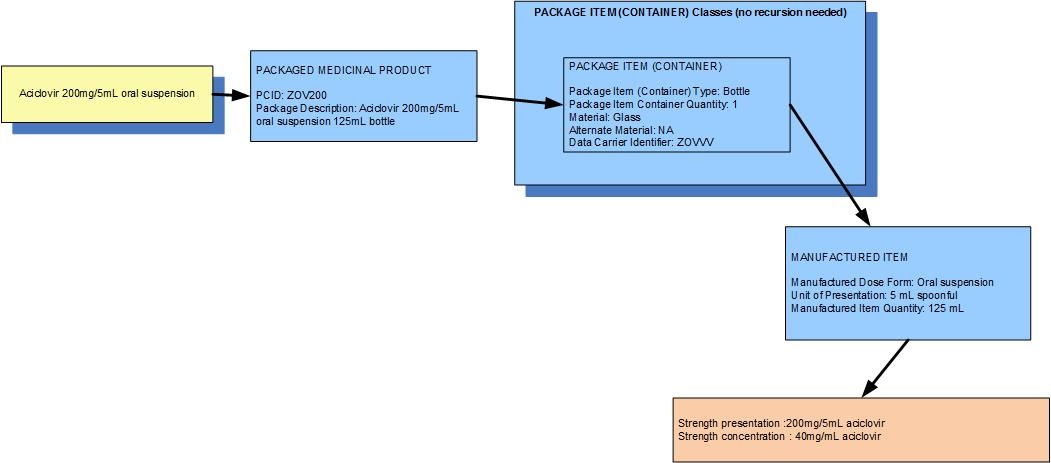
| Manufactured dose form | Oral suspension |
|
| Unit of presentation | 5 mL [spoonful] |
|
| [Pack size] | 125 mL in the bottle | Not usually expressed as 25 spoonfuls! |
| Precise active ingredient | aciclovir |
|
| Basis of strength substance | aciclovir |
|
| Presentation strength (logical) | 200 mg per 1 unit of presentation |
|
| Presentation strength | 200 mg per 5 mL |
|
| Concentration strength | 40 mg per 1 mL |
|
Examples: vials, ampoules, sachets, containing solid dose forms such as powders or granules which may or may not be dissolved before administration
The unit of presentation usually either uses the same word (even though it is a different concept) as the (package item) container, or the (package item) container is a more granular concept and the unit of presentation uses a less granular term.
Strength is expressed as "per one unit of presentation" and the presentation strength and the concentration are exactly the same.
Example 1: A pack of 10 vials each containing 2g cefotaxime powder for solution for injection
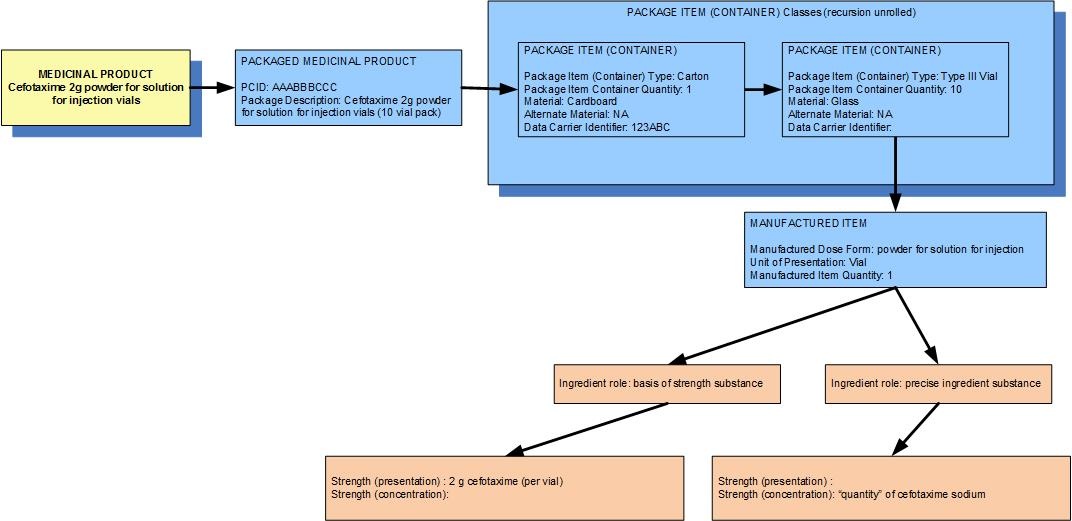
| Manufactured dose form | Powder for solution for injection |
|
| Unit of presentation | vial | The vial "bounds" the 2g of the dose form |
| [Pack size] | 10 vials in the carton |
|
| Precise active ingredient | cefotaxime sodium |
|
| Basis of strength substance | cefotaxime |
|
| Presentation strength (logical) | 2 g per 1 unit of presentation |
|
| Presentation strength | 2 g [per 1 vial] | UCUM: 2 g per 1 each |
| Concentration strength |
| The concentration of cefotaxime in the powder inside the vial is known to the regulatory agency but is not deemed clinically significant |
Example 2: A pack of 50 sachets containing 4g of colestyramine powder for oral solution
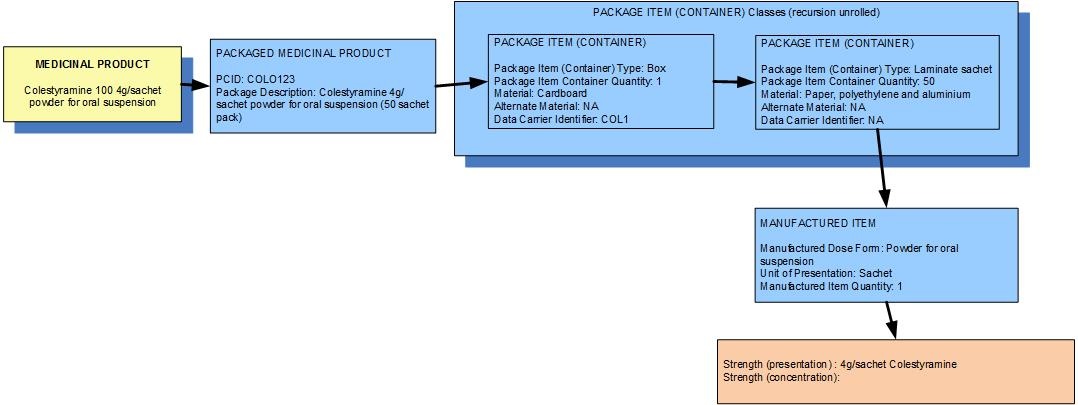
| Manufactured dose form | Powder for oral suspension |
|
|---|
| Unit of presentation | Sachet | The sachet "bounds" the 4g of the dose form |
|---|
| [Pack size] | 50 sachets in the box |
|
|---|
| Precise active ingredient | colestyramine |
|
|---|
| Basis of strength substance | colestyramine |
|
|---|
| Presentation strength (logical) | 4 g per 1 unit of presentation |
|
|---|
| Presentation strength | 4 g per 1 sachet | UCUM: 4 g per 1 each |
|---|
| Concentration strength |
| The concentration of colestyramine in the powder inside the sachet is known to the regulatory agency but not deemed clinically significant |
|---|
Examples: parenteral solutions, unit dose nebuliser solutions
The unit of presentation usually either uses the same word (even though it is a different concept) as the (package item) container, or the (package item) container is a more granular concept and the unit of presentation uses a less granular term.
Presentation strength is expressed as "per the amount of liquid bounded by the unit of presentation" but concentration strength is per mL (and therefore is often different).
Example 1: A pack of 10 ampoules each containing 20mL of metoclopramide hydrochloride for solution for injection
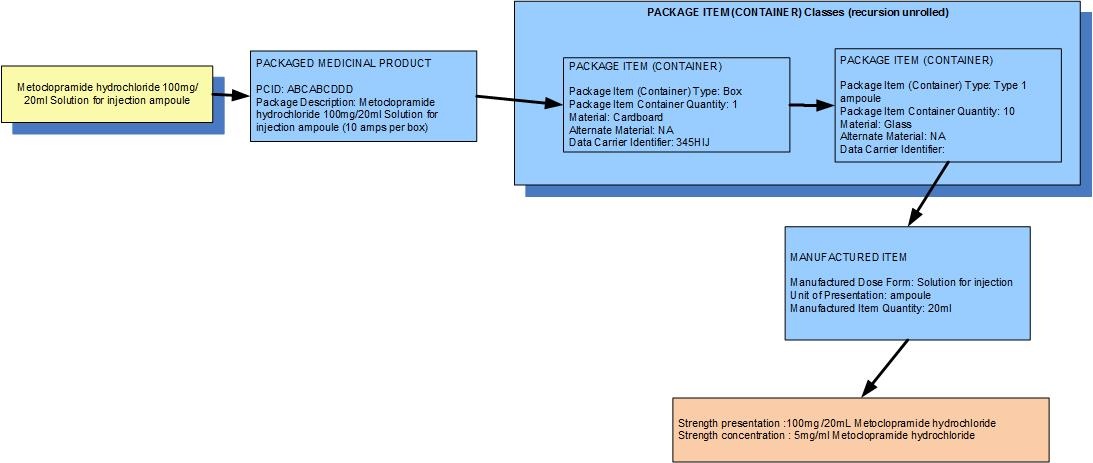
| Manufactured dose form | Solution for injection |
|
| Unit of presentation | Ampoule | The ampoule "bounds" the liquid |
| [Pack size] | 10 ampoules in the box |
|
| Precise active ingredient | metoclopramide hydrochloride |
|
| Basis of strength substance | metoclopramide hydrochloride |
|
| Presentation strength (logical) | 100 mg per volume contained in the unit of presentation | The amount of the dose form bounded in the unit of presentation |
| Presentation strength | 100 mg per 20 mL |
|
| Concentration strength | 5 mg per 1 mL |
|
Example 2: A pack of 20 UDVs each containing 2mL of ipratropium bromide for solution for nebulisation
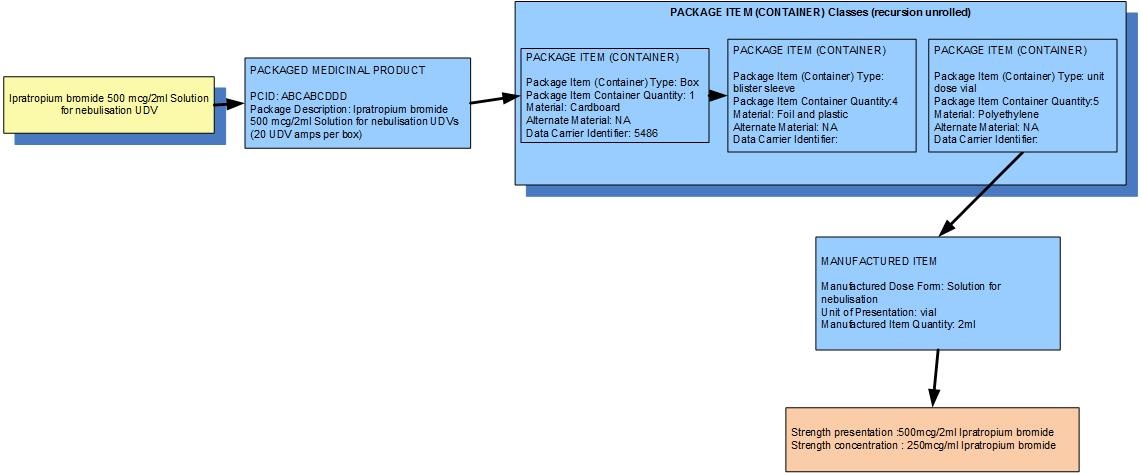
| Manufactured dose form | Solution for nebulisation |
|
| Unit of presentation | Unit dose vial | The vial "bounds" the liquid |
| [Pack size] | 5 vials in a sleeve, 4 sleeves in the box |
|
| Precise active ingredient | ipratropium bromide |
|
| Basis of strength substance | ipratropium bromide |
|
| Presentation strength (logical) | 500 mcg per volume contained in the unit of presentation | The amount of the dose form bounded in the unit of presentation |
| Presentation strength | 500 mcg per 2 mL |
|
| Concentration strength | 250 mcg per 1 mL |
|
Examples: bulk parenteral solutions, insulins, patches
The unit of presentation usually either uses the same word (even though it is a different concept) as the (package item) container, or the (package item) container is a more granular concept and the unit of presentation uses a less granular term.
However, although presentation strength is expressed as "per one unit of presentation" the clinically relevant strength is the concentration strength as almost all are used in individually calculated and variable amounts.
Note that the unit of presentation is likely to be useful in description of the medicinal product concept at some level; insulin presented in a multi-dose vial will be used/administered differently from insulin presented in a cartridge for use within a "pen device".
Example 1: A pack of 5 cartridges each containing 1.5mL soluble human insulin 100 units/mL solution for injection
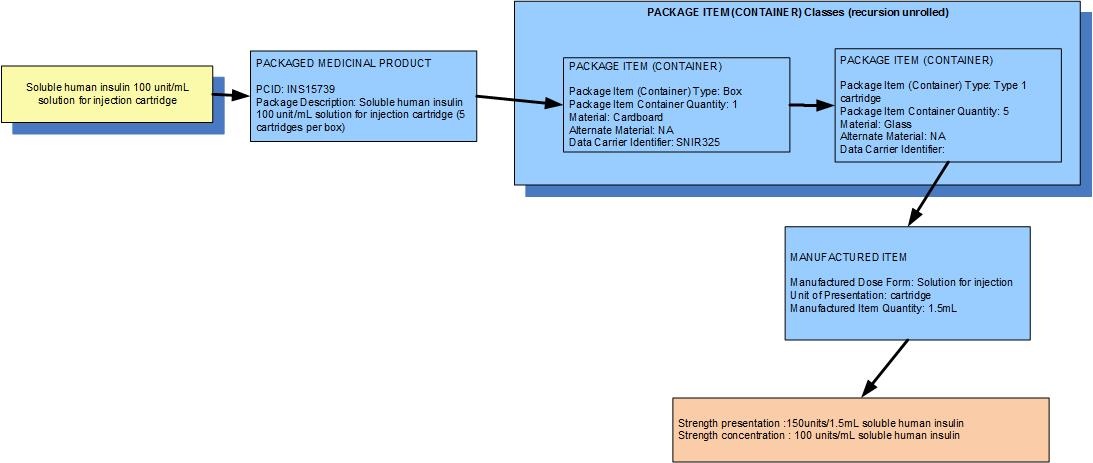
| Manufactured dose form | Solution for injection |
|
| Unit of presentation | Cartridge | The vial "bounds" the liquid |
| [Pack size] | 5 cartridges in a sleeve,1 sleeve in the box |
|
| Precise active ingredient | insulin soluble human |
|
| Basis of strength substance | insulin soluble human |
|
| Presentation strength (logical) | 150 units per volume contained in the unit of presentation | The amount of the dose form bounded in the unit of presentation |
| Presentation strength | 150 units per 1.5 mL | Not a clinically safe expression of strength |
| Concentration strength | 100 units per 1 mL |
|
Example 2: A box of 10 bags of Sodium chloride 0.9% solution for infusion 500mL
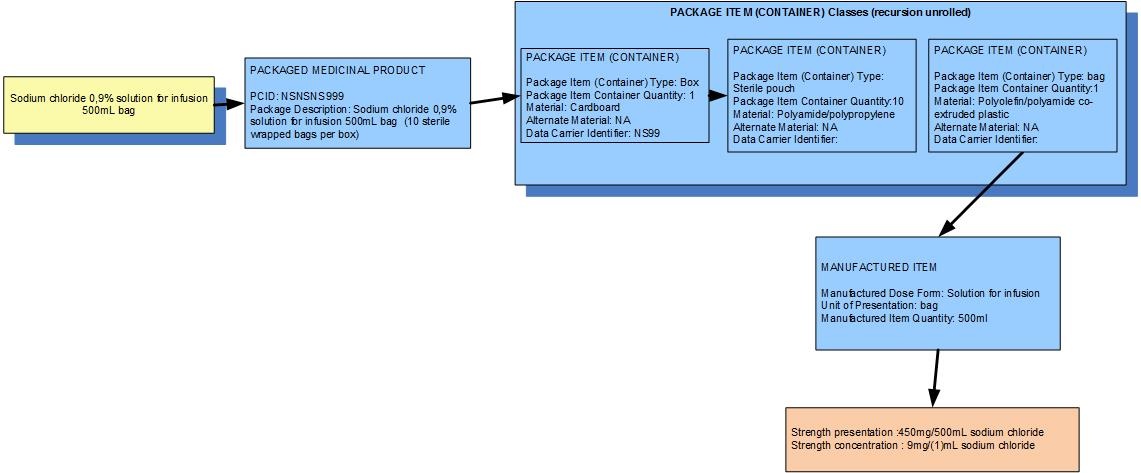
| Manufactured dose form | Solution for injection |
|
| Unit of presentation | Bag | The bag "bounds" the liquid |
| [Pack size] | 1 bag in a sterile pouch,10 pouches in the box |
|
| Precise active ingredient | sodium chloride |
|
| Basis of strength substance | sodium chloride |
|
| Presentation strength (logical) | 450 mg per volume contained in the unit of presentation | The amount of the dose form bounded in the unit of presentation |
| Presentation strength | 450 mg per 500 mL | Not a clinically safe expression of strength |
| Concentration strength | 9 mg per 1 mL | Synonym: 0.9% w/v |
Example 3: A pack of 30 sachets each containing a transdermal patch delivering 4.6mg per 24 hours of rivastigmine
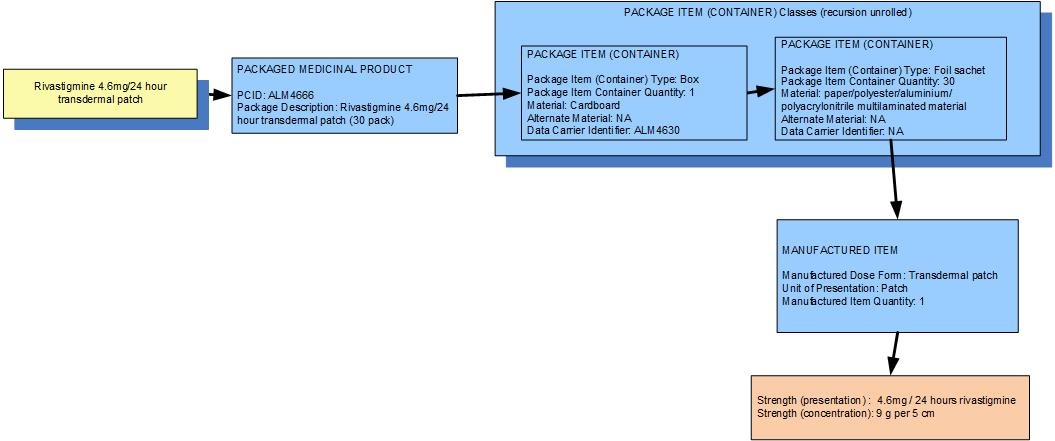
| Manufactured dose form | Transdermal patch |
|
| Unit of presentation | Patch | The patch "bounds" the dose form that delivers the medication |
| [Pack size] | 1 patch in sachet, 30 sachets in the box |
|
| Precise active ingredient | rivastigmine |
|
| Basis of strength substance | rivastigmine |
|
| Presentation strength (logical) | 9 g per volume contained in the unit of presentation | The amount of the dose form bounded in the unit of presentation |
| Presentation strength | 9 g per (5cm) patch | Not a clinically safe expression of strength |
| Concentration strength | 4.6 mg per 24 hours | This is a "rate" strength |
Continuous presentation: unbounded by container
Examples: bulk powders and granules, bulk liquids, semi-solids
These presentations are not particularly bound by their container in any way that is meaningful in terms of their use or administration; the Manufactured Item is a continuous presentation and almost all are used in individually calculated and variable amounts. For example, hydrocortisone cream for cutaneous use is contained in a tube, but its use is based on how much is squeezed out and applied to the skin; chloramphenicol eye drops are presented in a dropper bottle, but they are administered drop by drop and although the dropper bottle aims to deliver a roughly uniform sized drop to the eye, they are not "metered dose containers" in the way that containers with valves are.
A unit of presentation is not usually given for these products. The pack size (not relevant for SNOMED international model) is given as the Manufactured Item quantity.
Strength is expressed as a concentration and as such the presentation strength and the concentration are exactly the same.
Example 1: A 30g tube of hydrocortisone 1% cutaneous cream w/w in an outer box
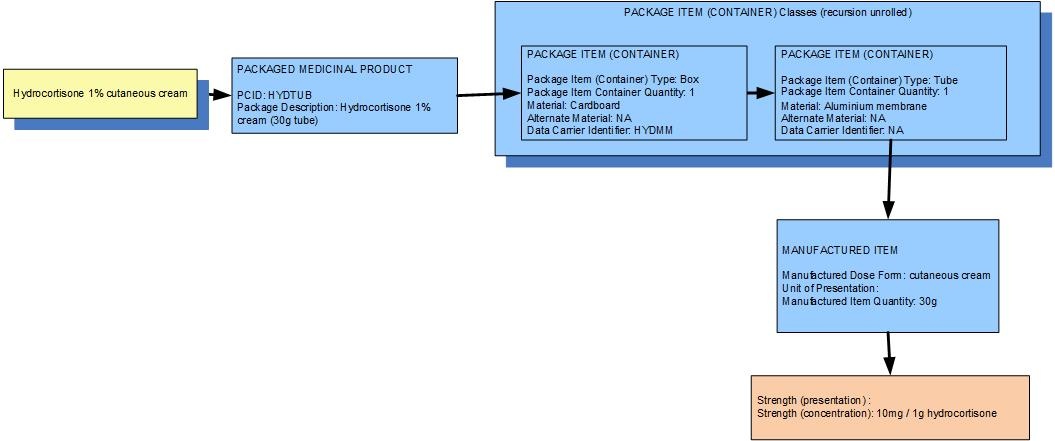
| Manufactured dose form | Cutaneous cream |
|
| Unit of presentation | NOT VALUED | There is nothing that really "bounds" the dose form that delivers the medication "per dose" |
| [Pack size] | 30 g in the tube,1 tube in the box |
|
| Precise active ingredient | hydrocortisone |
|
| Basis of strength substance | hydrocortisone |
|
| Presentation strength (logical) | 300 mg per 30 g | Not a clinically safe or recognisable expression of strength |
| Presentation strength |
| Concentration strength | 10 mg per 1 g | Synonym: 1.0 % w/w |
Example 2: A bottle of 5 mL of chloramphenicol eye drops 0.5% w/v in an outer box
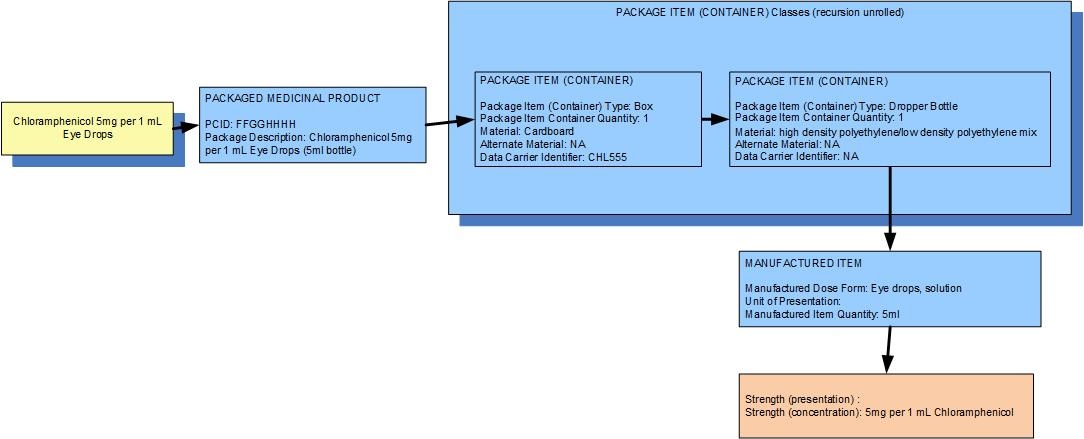
| Manufactured dose form | Eye drops, solution |
|
| Unit of presentation | NOT VALUED | There is nothing that really "bounds" the dose form that delivers the medication "per dose" |
| [Pack size] | 5 ml in the dropper bottle,1 bottle in the box |
|
| Precise active ingredient | chloramphenicol |
|
| Basis of strength substance | chloramphenicol |
|
| Presentation strength (logical) | 25 mg per 5 mL | Not a clinically safe or recognisable expression of strength |
| Presentation strength |
| Concentration strength | 5 mg per 1 mL | Synonym: 0.5 % w/v |
Example 3: A pack of 500g of sterculia 62% w/w granules (EDQM –"granules" = "oral granules")
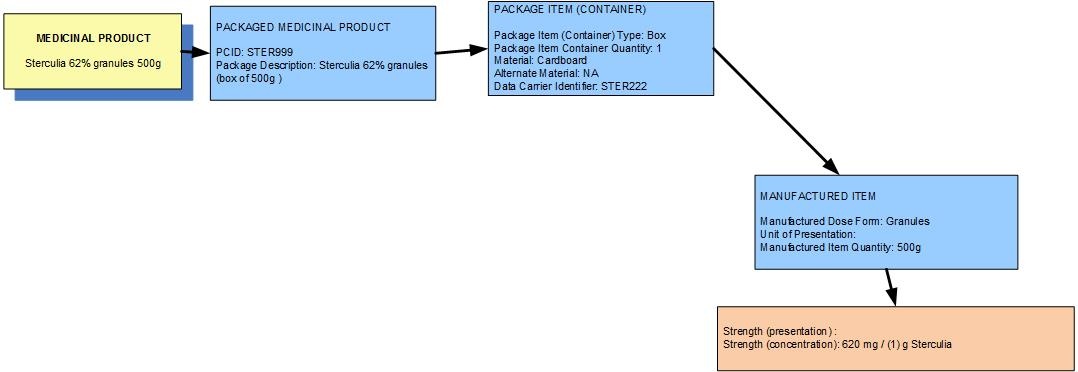
| Manufactured dose form | Oral granules |
|
| Unit of presentation | NOT VALUED | There is nothing that really "bounds" the dose form that delivers the medication "per dose" |
| [Pack size] | 500 g in the box |
|
| Precise active ingredient | sterculia |
|
| Basis of strength substance | sterculia |
|
| Presentation strength (logical) | 310 g per 500 g | Not a clinically safe or recognisable expression of strength |
| Presentation strength |
| Concentration strength | 620 mg per 1 g | Synonym: 62 % w/w |
Example 4: A bottle of 60 mL of digoxin oral solution 50mcg/mL in an outer box (which has an oral syringe in it)
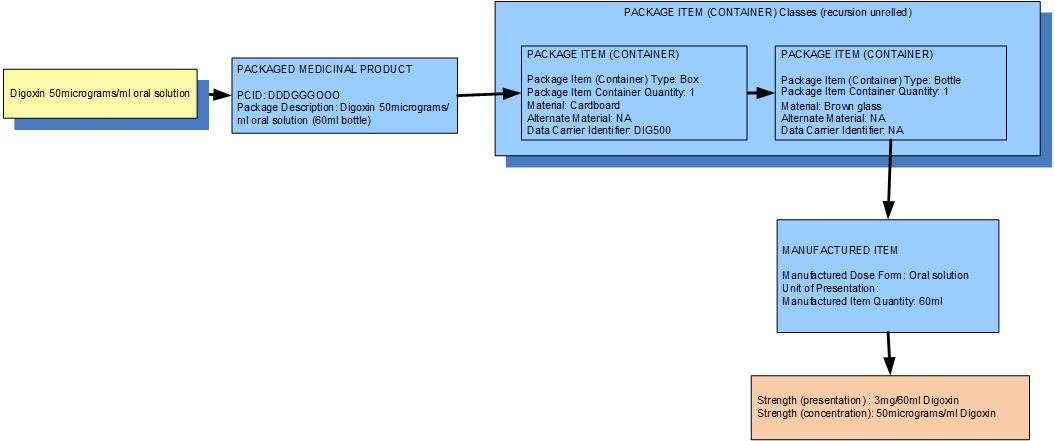
| Manufactured dose form | Oral solution |
|
| Unit of presentation | NOT VALUED | There is nothing that really "bounds" the dose form that delivers the medication "per dose" |
|
| [Pack size] | 60 ml in the bottle,1 bottle in the box |
|
|
| Precise active ingredient | digoxin |
|
| Basis of strength substance | digoxin |
|
| Presentation strength (logical) | 3 mg per 60 mL | Not a clinically safe or recognisable expression of strength |
|
| Presentation strength |
| Concentration strength | 50 mcg per 1 mL | Synonym: 0.5 % w/v |
|















