Page History
Overview
Using the Any SNOMED CT subsets for subset used to code COVID-19 data coding is dependent on a range of factors related to the local requirements and context of usage. Overall, the following three steps are important when planning and preparing the use of these subsets. In the following sections, we will provide information on each of these steps.
Identify Data Elements
should be designed based on its purpose and the context in which it will be used.
To make the process of planning and designing your SNOMED CT subsets more efficient and internationally consistent, we recommend the following three steps.
These three steps are explained in more detail below.
Step 1 - Identify Data Elements
The first step is to identify the data elements that may require SNOMED CT coding. These data elements may already be defined in national reporting requirements Footnote ref Footnote ref n 2
, as part of a healthcare data standard you are using (e.g. a HL7 FHIR profileFootnote ref n 3
or openEHR archetypesFootnote ref n 4
), or you may be developing a new information model (or data set) for this purpose. Your requirements for coded content will depend on your specific use case and the data items in your information model. In additionAlthough the recording and use of COVID-19 data revolve around the same clinical scope, the requirements for content depends on the specific use case and the involved data items. Furthermore, different countries, regions, and hospitals may apply different clinical techniques or practices, which can also result in differing coding requirements for content. Therefore Therefore, deciding which subsets to implement require you to clarify requires clarification of the scope of the content requiredneeded, by answering questions such as:Footnote ref n 5
- What will the your SNOMED CT subsets be used for?
- Will it they be used to capture new data in a clinical information system to support frontline service delivery?
- Will it they be used for disease surveillance?
- Will it they be used to integrate data from various sources?
- Will they be used for international collaboration?
- Will they it be used for retrospective analysis of data?
- Which data elements are required for the your use case?
- For data capture, what What coded data needs to be collected to support the clinical processcare?
- For disease surveillance, what parameters are required to monitor the disease progression?
- For data integration, what data items will be shared or communicated?
- For retrospective analysis of data, what are the available data items that are relevant to include for SNOMED CT analytics?
- What coded data needs to be collected to provide disease surveillance?
- What coded data needs to be shared between or integrated from different sources?
- What coded data is required for international collaboration?
- What coded data may be needed for retrospective data analysis?
- What existing subsets are available for the data elements of interest?
- Where these subsets designed based on the same or compatible requirements
- Are the subset members adequate for our needs?
Answering these questions will help in understanding which of the available existing subsets are relevant for your context, and it will also bring attention to . It may also help to identify potential content gaps in the subsets, or extraneous concepts which may be unnecessary for the are not required for your specific scenario. Please note that the questions above are provided for inspiration , and there only. Additional questions specific to your use case are likely to be additional questions to answer specific to your contextneeded.
Step 2 - Download Subsets
Subset Categories
The SNOMED CT COVID-19 subsets have been developed as a collection of concepts contributed from a range of Members that are considered to be potentially relevant to COVID-19 data coding. The subsets have been organized into the following 5 categories, determined by the data items which are likely to be recorded together.
Patient Demographics (PAT)
Clinical Assessment (ASS)
Tests and Investigations (INV)
Please follow the links in the lists above for detailed information on the specific subsets within each category.
Reference Set Tool
The SNOMED CT COVID-19 subsets are made available via the SNOMED International Reference set and translation tool, which can be accessed via this link: https://refset.ihtsdotools.org/
The subsets are placed in the 'COVID-19 subsets' project and are named according to the naming convention which is presented below.
| Info |
|---|
The IHTSDO Reference Set Management and Translation Tool provides a directory of existing published content reference sets and translations, currently against the SNOMED CT International Edition. If you would like to request an account to create your own reference sets or translations, please contact techsupport@ihtsdo.org. |
Subset Types
Some of the subsets are developed
| Gloss | ||||
|---|---|---|---|---|
|
| Gloss | ||||
|---|---|---|---|---|
|
For the subsets that have been extensionally defined, we provide two versions:
- One version which only includes the members listed
- One version which includes the members listed, and the descendants of each of these members. This version supports the collection of more specific values where these are available.
Naming Convention
The second step is to download any available existing subsets that are associated with your required data elements.
The example COVID-19 subsets in this guide can be downloaded from Appendix A - Example Subsets.
Subset Naming Convention
The example COVID-19 subsets provided by SNOMED International have been named using the following conventionThe reference sets have been named using a naming convention which follows the general categories of subsets.
| Info | ||
|---|---|---|
| ||
|
Please see the examples of subset naming in the table below.The table below shows some examples of subsets named using this convention.
| Examples of Named Subset |
|---|
| Category | Subset | Name |
| Provider and Facility Details ( |
| PFD) | Site of |
| care subset | CV19- |
| PFD-SiteOfCare | ||
Patient Demographics (PAT) | Marital status subset | CV19-PAT-MaritalStatus |
| Marital status subset including subtypes | CV19- |
| PAT-MaritalStatus-withSubtypes | ||
Clinical Assessment (ASS) | Symptoms subset | CV19-ASS-Symptoms |
| Symptoms subset including subtypes | CV19-ASS-Symptoms-withSubtypes |
Tests and Investigations (INV)
Subset Categories
The international SNOMED CT subsets have been organized into a number of categories, based on groupings of data elements that are likely to be recorded together. The five categories identified are:
Patient Demographics (PAT)
Clinical Assessment (ASS)
Tests and Investigations (INV)
...
Please follow the links above for information on the specific subsets within each category.
Subset Types
Some of the international subsets have been developed intensionally, while others have been developed extensionally. Please refer to the Practical Guide to Reference Sets, section 2.1.1. Subset Definitions for information on the difference between intensionally and extensionally defined subsets.
For each extensionally-defined subset, where the members have subtypes, we provide two versions:
- One subset which includes only the listed members. This version supports use cases (e.g. reporting, data integration) that require more abstract concepts.
- One subset which includes the members listed and all the subtypes of each of these members. This version supports use cases that may require more specific values (e.g. data collection where more clinical detail is required).
- Please note that this second type of subset is automatically generated from the corresponding subset with listed members. No manual curation has been performed, and therefore these subsets may contain concepts that are not relevant for the context of use.
Step 3 - Review and Adapt
The third step is to carefully review each international subset, and adapt it to meet your specific requirements. The subsets provided represent a collection of concepts that have been
Review and Adapt
Prior to deployment, the subsets should be carefully reviewed and adapted to meet national or local requirements. As the subsets represent collections of concepts contributed from a range of SNOMED International Members, it . It is therefore important to review the each subset members to identify member to:
- Remove any concepts that are not
...
- required for your use case
- Add any concepts missing from the subset that may be required for your specific use case
- Please note, if the concept you require is not included in the SNOMED CT Edition you are using, please follow the content request processes in each country. For more information, refer to the relevant SNOMED International Member page.
- SNOMED International National Release Centers and other authorized users may request additions or changes to the SNOMED CT International Edition via the SNOMED CT Content Request Service. For more information, refer to the CRS User Guide or contact info@snomed.org.
- Remove any concepts that are not included in the SNOMED CT versioned edition being used. This may involve
- Checking the effective time of each international concept to ensure that it is less than or equal to the International Version used by your local SNOMED CT edition
- Checking that any extension concepts are published in the SNOMED CT edition you are using
- Ensure that your subset aligns with best practice principles for subset creation.
- For example, this may involve checking that all members of the subset belong to a single hierarchy (in most cases), and that no two members subsumesubsume each other (in most cases).
- For more information, please watch our e-learning presentation on this topic - Subset Creation Principles
...
- .
Unpublished Content
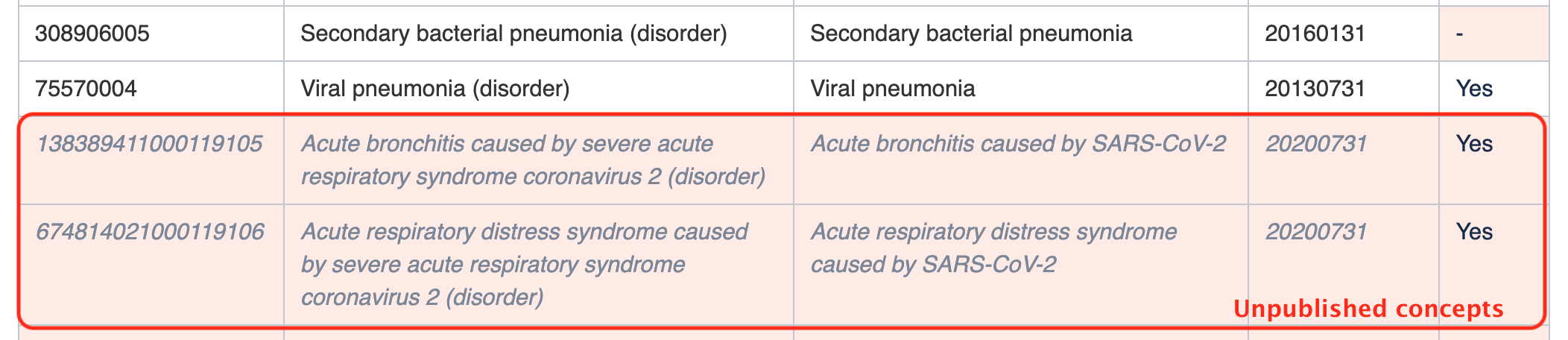
Please note that the subsets in this guide may include some unpublished content , that that is planned for a future SNOMED CT edition. This content should not be used until it has been published. An example of such member is the concept
in the CV19-ASS-Complications subset. This concept will be added in the July 2020 version of the International Edition. Although they are not yet available in the downloadable version of the subset, they are referenced in the subset tables of this guide to bring the upcoming additions to your attention and assist with planning.officially published, as we cannot guarantee that the identifiers or terms will not change. For this reason, these unpublished concepts are not included in the downloadable versions of the subsets. Unpublished concepts are included in this guide to assist with planning (e.g. planning of national extensions).Concept t 882784691000119100 | Pneumonia caused by severe acute respiratory syndrome coronavirus 2 (disorder) |
Unpublished content is shown in this guide using grey, italic font on a pink background, as shown in the example below.
Extension Content
In response to an immediate need for COVID-19-specific concepts, various Member countries have developed concepts within their national SNOMED CT Extensions. These Some of these extension concepts are documented in this guide and may be used as an inspiration to other Members. All though, please note that the extension concepts for COVID-19 are not included in the International Edition, and any implementers must only use the extension concepts available within the Edition they deploy. The extension concepts are not available in , for the interest of other Members, see 4. Extension Content for COVID-19. Please note, however, that extension concepts should only be used when the module in which they are published is included in the implemented SNOMED CT Edition. SNOMED CT extension concepts are not included in the subsets downloadable from the Reference Set tool.
Feedback
We welcome any comments, feedback, and suggestions.
SNOMED International welcomes comments on this guide and suggestions for new or updated content. Please use the Feedback button at the bottom of each page to send us your feedback.
| Footnote block |
|---|
|
...