Date 20171017
0700-1500 UTC
0900-1700 Bratislava time
0500-1300 Eastern Daylight Time
Zoom Meeting Details
SNOMED Int'l Editorial Advisory group
SNOMED International - Editorial advisory group conference call
UTC
Please join my meeting from your computer, tablet or smartphone.
Attendees
Chair:
AG Members
Observers:
- Monica Harry
- Krista Lilly
- Maria Braithwaite
- Donna Morgan
- Maggie Lau
- Ismat Mohd Sulaiman
- Toni Morrison
- Eric Rose
- Cathy Richardson
- Elze de Groot
- Katrien Scheerlinck
- Nicola Ingram
- Regis Charlot
- Phuong Skovgaard
- Gary Dickinson
- Timothy Williams
- Emma Melhuish
- Alejandro Lopez Osornio
- Matt Cordell
- Penni Hernandez
- Candy Barth
- Rita Barsoum
- Corey Smith
- Scott Campbell
- Mary Kennedy
- Raj Dash
- James R. Campbell
Objectives
- Obtain consensus on agenda items
Discussion items
| Item | Description | Owner | Notes | Discussion | Action |
|---|---|---|---|---|---|
| 1 | Call to order and role call | JCA | |||
| 2 | Conflicts of interest Approval of minutes 20170928 | JCA |
| ||
| 3 | Continued from 20170928: Change of name for genetic diseases | JCA | Based on requests from UKTC: The concepts are The FSN for these concepts align with Orphanet, OMIM and Genetics Home Reference. The request from the UKTC is All terms should ideally be replaced by autosomal dominant tubulointerstitial kidney disease (ADTKD) (see KDIGO report). The above terms are not necessarily the same and don’t really reflect the improved clinical descriptions of the disease based on genetics. ADTKD reflects the inheritance, common phenotype caused by different mutations and can be used for suspected cases. This is well described in the KDIGO report. They also make the point it is a simple term to use and that MCKD is frankly inaccurate! As above. I would favour not using these terms MCKD 1 and 2 even though they may be commonly used at present. ADTKD-UMOD or ADTKD-MUC1 would be the preferred names. The list of genes is also increasing making a single term more appropriate. ADTKD would be the parent and the children would be ADTKD associated with UMOD mutations and ADTKD associated with MUC1 mutations. It is anticipated that this type of request will become more frequent as the move towards genomics continues. Question: Do we go with the current naming convention to align with Orphanet (our current "Source of truth") or try to keep pace with the evolving nature of content in this area? 10/6/2017: Response from Orphanet After checking, I confirm the proposed modification of nomenclature from your contact. These modifications don't change the concepts nor the current mappings. ORPHA34149 Autosomal dominant tubulointerstitial kidney disease (Disease) Question: Do we change the FSN or inactivate and replace? In this case it is clear from the response that the "meaning" of the concept is unchanged. For organisms, we have adopted the policy that when taxonomic names change, it is not the organism that changes, but the term representing the organism, thus we rename the FSN for the concept and retain the "older" term as a historical synonym as the naming transition for searching convenience. Should we adopt the same policy for disorders, or does this constitute a substantive change compelling us to inactivate and replace? | Summary of past discussion:
Orphanet release cycle (from Maria Braithwaite): Orphanet have an ongoing cycle of release for new definitions and changes to the website, they do not currently routinely inform me of a change to the name of a particular entry but I will ask them if it is possible to provide this information. We agreed that they will provide me with a list of changes (new additions, deprecated or obsolete entries) twice per year in April and October to allow me to make content edits before we close the release. This will prevent problems I have had previously where a new concept has been published almost simultaneously with Orphanet deprecating their entry. 10/17/2017 Discussion: Suggested that we need more specific written guidance on what constitutes "change of meaning". Until that time, the safest thing to do is to retire and replace. Also suggested that the old term be retained as a description associated with the new concept. The example of the policy used for organism name changes was presented, but this is a primitive hierarchy. In the cases discussed here, there is no change to the underlying modeling, only a name change. Terms that are inherently vague or ambiguous that are clarified by name changes or additional relationships would mandate inactivation and replacement. Additional documentation: http://pediatrics.aappublications.org/content/early/2016/04/21/peds.2016-0590 Do we need a new inactivation status that reflects the inactivation due to a change in understanding of the concept? i.e. refined knowledge? |
|
| 4 | Demo: Batch structural changes to existing content | GRE | Brief demonstration of the tooling that will be used to revised the inconsistencies identified in the structure of SNOMED CT content. Examples of the types of patterns that will be addressed can be found at: http://qa.snomed.org/ | ||
| 5 | ECE Update | BGO |
The third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) published in 2016 state sepsis is a multi organ dysfunction syndrome due to an infection or more specifically due to an dysregulated host response to infection. Current model places sepsis as a subtype of SIRS and infectious disease which is not consistent with Sepsis-3 definition. Proposed model: IsA Multiple organ dysfunction syndrome due to infection. Question: Would a new pathological process of dysregulated host response be required in order to fully define sepsis? | BGO presented the discussion form the ECE meeting the previous day. See slides from Sepsis models.pptx atttached. Is the shift in meaning from the current representation in SNOMED CT going to cause issues for users if we change it? Agreement that the current modeling is wrong? Discussion as to the need for the new pathological process, i.e. does it add value to the definition? Suggested that there is a need for another PATHOLOGICAL PROCESS "Abnormal immune response". GRE Brought up the history of the inconsistency of use of PATHOLOGIC PROCESS. This lead to a severe restriction on its range. Discussion on expanding its range and how the editorial guidance can be tightened to ensure consistency. Group agreed that Sepsis should be remodeled according to the new definition. GRE mentioned with the introduction of multiple sufficient sets, we can support the transition. |
|
6 | Findings related to skin wounds | JCA | A number of requests related to findings related to surgical skin wounds and pressure injury findings reveal an issue with current structure. Most of the requested terms are Findings related to skin wounds, but currently 262526004 |Wound of skin (disorder)|is a disorder, so cannot be used as a parent for findings related to skin wounds. There is currently 225552003 |Wound finding (finding)|, but it is not specific to skin. 262526004 |Wound of skin (disorder)|currently has 65 immediate subtypes, many of which could reasonably be viewed as findings (e.g. “Abrasion of X”). Need to make a determination of whether observations related to wounds (i.e. color, discharge, odor) should be placed in a subhierarchy different from the "Wound (disorder)" itself. | Agreed that wounds are not disorders per se and findings about wounds should be classified under wound finding. |
|
| 7 | Specimen from subjects other than the patient | JCA | Currently we have many concepts in the specimen hierarchy that include “from patient”as well as those that do not include it as an ancestor. Since the subject of record is the default for specimens, we would like to retire these apparent duplicates, but then we run into the problem of specimens derived from other sources such as donors or normal control patients. They cannot be subtypes if the intended meaning is “subject of record”..or can they, since the context is implied? How do we structure the specimen hierarchy to account for this? What are the analytical implications of having different sources for specimens as subtypes of one another? | The "soft" default of specimens originating from the patient is where the problem lies. Currently, the organization of the values for SPECIMEN SOURCE causes some specimens from patients to not classify under the grouper term "Specimen from patient". Suggestion to make a more general term "Patient from specimen or donor", but that would only address two of the SPECIMEN SOURCE types. KCA mentioned the many issues with the "soft default'. For the most part, these specimens are used as coming from the patient. However, does the FSN then need to be changed to reflect that these are from the patient? Does the PT need to reflect explicitly that it comes from the patient? Should every Specimen have the SPECIMEN SOURCE explicitly defined? Often this context comes from where the concept is used within the record. The history of these terms may provide some of the reasoning as to why these terms were created. For example, the restrictions in where codes could be used in earlier versions of HL7 v2 (i.e. prior to v2.5) meant there was not place to provide additional information around the specimen. KCA suggested that these then be segregated into a module so that they can eventually be segregated away from the core. Non-patient oriented specimens are the major issue now and many of the use cases still use the older transport structure, meaning they need pre-coordinated content. What are the requirements for the addition of these terms and what is the major function of the core to address these requirements? More modern transport mechanisms such as FHIR, do not need this level of pre-coordination. Comments from IMO indicated that most users of the terminology are not sufficiently sophisticated to use either terminology or model based post-coordination. The long-standing practice of using unspecified Specimens provides substantial challenges to revising this to make it explicit as it would result in a large number of changes that may impact implementers. | |
| 8 | WAS-A Inactivation redux | JCA | Concerns have been expressed about the impending inactivation of existing WAS-A relationships: "This topic has consulted with the CMAG and UKTC. The feedback from CMAG was that this should not be a priority. The size and efforts are small for content maintenance. The potential impact could be high if we make changes. The feedback from UKTC was to delay the changes until 2018 when they move to RF2. Furthermore, they still think it would be useful to provide information for WAS A by technical means centrally. " | Jeremy Rogers presented the use case for these terms within the UKTC. Guillermo Reynoso Described the history of WAS A relationships. The observation was made that these relationships have not been updated for a number of years so do not represent the full scope of inactivation relationships. The WAS A relationship is no longer available and was primarily used to model "limited" concepts, which were made inactive in 2010. The ability to segregate these from the core using a module approach was also suggested. Also suggested that these be moved back to the UK extension so that they have full control over how to use them as they are not needed by other extensions. It was suggested that if there is still a need to have access to WAS A relationships for transitive closure, then a complete set of these can be reconstructed from the RF2 files. which would be more complete than the current set. There was also discussion about the ambiguity of REPLACED BY, which is also no longer used. |
|
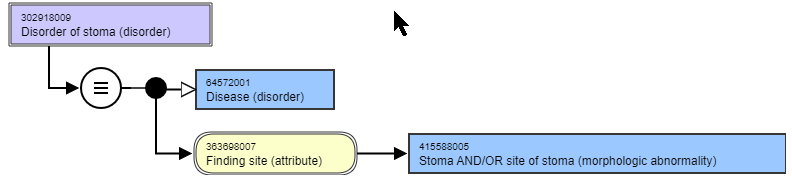
| 9 | Morphologic abnormalities as values for FINDING SITE | JCA | This arose during a review of "Disorder of stoma (disorder)" Currently there are 16 disorders and 23 findings that have a value of 245857005 |Stoma (morphologic abnormality)|. As the stoma is a morphologic structure within a body structure, is it legitimate to allow this as a finding site? For the most part the terms that use this value are nonspecific to the site of the stoma. Additionally, it is unclear what the use of 91241007 |Stoma site (morphologic abnormality)|, given that the site of a stoma can be values using any anatomical site. Questions:
| Suggestion is that a stoma is not a morphology, but is an "Acquired body structure". The current descendents of "acquired body structure" include a number of morphologic abnormality concepts. There is cleanup needed in this subhierarchy. There are a number of post-surgical structures or procedural structures that have been given the semantic tag of "morphologic abnormality". If these were cleaned up, then they could be used as values for the FINDING SITE more clearly. Proposed to review the existing concepts that use morphologic abnormalities as the value for FINDING SITE to determine whether they can be added as subtypes of "acquired body structure", Those that are appropriate will have the semantic tag changed to "body structure". The MRCM will need to be revised to disallow "morphologic abnormalities" from being the value of FINDING SITE |
|
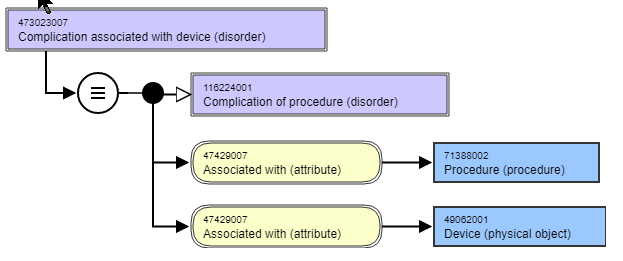
| 10 | What is an "infected prosthesis" | JCA | We have a number of terms, both disorder and procedure that deal with "infected prosthesis". In general, prostheses themselves are not infected, but the surrounding soft (or bone) tissue adjacent to the prosthesis can become infected. This infection often does not have a demonstrable causal or temporal relationship to the procedure. Currently these are modeled with an ASSOCIATED WITH relationship: Question: How do we best represent the true nature of the infection? This is especially important when we deal with "Removal of prosthesis due to infection" and concepts such as "Infection of implanted cardiac device (disorder)".? Based on previous discussions regarding "causal chain", should this be a DUE TO relationship since the infections would not have occurred if the procedure had not been done? | A prosthesis can be infected (e.g. vegetation on a prosthetic heart valve). The need to associate a procedure with these would be unnecessary and in many cases incorrect. The use of a DUE TO relationship is not appropriate. Currently, the involved concepts inherit ASSOCIATED WITH = Procedure from the parent "Complication associated with device", which should not have this relationship. There are also timing aspects that are not represented in these terms, which make them more vague. The associated problem is the need for a definition of what is meant by "infected device". If we view the presence of the device as just another acquired body structure, then these may not be complications. The timing of the infection in relation to a procedure, may be the reason to classify something as a Complication of a procedure (i.e. within a certain number of days). The two approaches are "close to reality", which is multi-dimensional and challenging to determine, or "simplified model" that just describes what is certain. The determination of whether something is a complication or not is often unknown. Some testing will need to be done to see the impact of applying a simplified model. If it does not meet the needs from a classification standpoint, then a more complex model will be needed. This argues for the use of ASSOCIATED WITH as the relationship for devices. For the procedures such as "Removal of prosthesis due to infection" the possibility of the use of HAS FOCUS. There are guidelines on the evaluation of patients prior to implantation, where pre-existing infection would cause abortion of the procedure. Clarification on the current understanding of Complications can be found here. |
|
| 11 | “Acquired” disorders vs. Congenital disorders | JCA |
| There are 690 "Acquired X" disorders in SNOMED CT. The vast majority are primitive. There are three alternatives to discuss:
This demonstrates a need to support disjointness, which will hopefully be supported by the concept model in the near future. There are challenges with using period of life as a way to classify these. What is the use case to make the distinction between congenital and acquired. Used the example of "anodontia". There is also the distinction between hereditary and "congenital", which are often conflated in disease naming. Hereditary diseases often manifest later in life. Would these be considered "acquired"? What do we mean when we say "acquired"? Could limit the use of the period of life grouper for only those disease where the FSN specifies "acquired". Would diseases that manifest later in life but are actually genetic be incorrectly classified under acquired disorder (if that were created)? Would there be an advantage of having this top level grouper? The challenge is how to represent the acquisition of the trait as opposed to the clinical manifestation of the trait. Suggested that a new qualifier value of "Post-natal" be created to aggregate the periods of life that would be used to define "Acquired" conditions. 2017-11-03: A related tracker exists: PCP-71. The work related to this item will be linked to that tracker. |
|
| 12 | Update of EAG Workplan | JCA | Review and revision of current workplan | Continued to next call due to lack of time. |
|
| 13 | Use of the Oxford comma in FSNs | JCA | The Oxford comma is a comma added after the penultimate term in a list, e.g. For example "Disorder of head, neck, and shoulders". The purpose if its use is to make explicit the fact that the terms are part of a list. The editorial guide is silent about its use, but the example provided does not use the Oxford comma. There are currently 347 FSNs in SNOMED CT that use the Oxford comma. Most of these are terms obtained from other terminology, such as ICD and nursing. There are 2500 FSNs that contain comma delimited lists, but do not use the Oxford comma. Question: Should SNOMED CT be consistent in the use of this grammar mark or maintain fidelity to the original source of the terms that do use it? | KCA expressed support for the Oxford comma. The question being whether there should be a retroactive application to FSNs. It does not change the meaning so would not be considered as a requirement for inactivation and replacement. JRO was not in favor of using the Oxford comma where it does not add value. The challenge is to provide editorial guidance on what the conditions are that require its use or non-use. | |
| 14 | AoB | Group | Placement of "conditions" and "predispositions" as clinical findings as opposed to disorders. - BGO Device disorder vs. device failure | Bruce Goldberg presented issues from the ECE meeting that required additional input: Complications and sequelae update.pptx Device complications Problems with the device itself should be a finding and not a disorder. This would allow some rearrangement of the current device problem findings. The modleing structure would be to use the INTERPRETS/HAS INTERPRETATION pair to define the findings. Should also create a more specific "device failure" to segregate from general external equipment failure. desire to see more examples for each of the three patterns. Hypersensitivity condition remodeling to finding: Predispositions are not disorders per se as they do not have a pathologic process. Proposed to move a large number of concepts from under disease to findings. Because the proposal is to simply change the semantic tag within the same hierarchy would not require inactivation and recreation of these concepts. The distinction between findings and diseases was brought up. The problems associated with this distinction and the duplication of terms as both findings and disorders was discussed. KCA asked to see a list of these duplicates |
|
| 15 | Future meetings | JCA | Next conference call TBD |